- Development of a dispersing agent to allow easy control of the dispersion and aggregation states of carbon nanotubes -
Masaru Yoshida (Leader) and Yoko Matsuzawa (Researcher), Smart Materials Group, the Nanosystem Research Institute (Director: Kiyoshi Yase) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have developed a new dispersing agent that allows easy control of the isolated dispersion and aggregation states of single-wall carbon nanotubes (SWCNTs) by UV irradiation, through the investigation of the molecular structures of agents.
Carbon nanotubes (CNTs), including SWCNTs, are attracting considerable attention as nanocarbon materials but are insoluble in solvents. The insolubility is limiting the applications of CNTs. In recent years, dispersing agents to disperse SWCNTs in solvents are being developed extensively at home and abroad. However, there was no established technology for precise control of the dispersion state of SWCNTs. The newly synthesized dispersing agent has high SWCNT dispersion ability and a chromophore (a photoreactive group). Because of the chromophore, the molecular structure of the agent can be changed by a UV-induced photoreaction, allowing easy removal of the agent from the SWCNT surface. This technology, which allows removal of a dispersing agent using a non-contact stimulus, is expected to improve the purifying techniques of SWCNTs and to have applications in the materials made of various types of CNTs.
Details of this technology will be published online in Advanced Materials on July 26, 2011.
 |
|
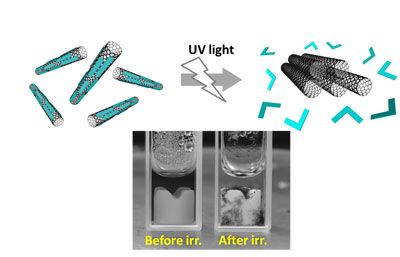
Figure : Schematic of the dispersing agent removed by light (shown in blue is the developed dispersing agent) (top)
and the SWCNT solution that underwent a change in dispersion state before and after UV irradiation
(before UV irradiation: dispersion, after UV irradiation: aggregation) (bottom)
|
In recent years, with the development of nanotechnology, the industrial use of single- and multi-wall carbon nanotubes as key materials is attracting attention (Strategic Technology Roadmap 2010 of the Ministry of Economy, Trade and Industry). One of the keys to such use is an advanced purification technology including separation techniques of semiconducting and metallic SWCNTs (AIST press release of May 11, 2011). In most purification techniques, it is very important to prepare a solution in which insoluble SWCNTs are isolated and dispersed using a proper dispersing agent. Although there are many such dispersing agents, there is no established technique for precise control of the dispersion state. Development of techniques to selectively remove the dispersing agent from the dispersion solution has been a major issue in the application of SWCNTs and other CNTs.
Through research on new dispersing agents, AIST has discovered that an organic compound with a cationic electrolyte structure shows high dispersion ability (AIST press release of May 25, 2007). It has worked extensively to develop new photofunctional materials and developed organic materials that change their state from solid to liquid by light irradiation without heating and can return to its original solid state (AIST press release of December 2, 2010). By combining these researches in different fields and introducing a photoreactive group into an organic electrolyte based on a new molecular design, AIST has conducted the research on the optical control of SWCNT dispersion, of which little knowledge had been obtained.
This study was partly supported by Grants-In-Aid for Scientific Research “Scientific Research (C) (FY2010-FY2012)” of the Japan Society for the Promotion of Science.
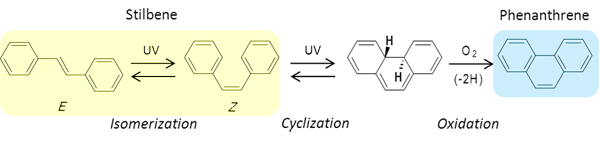
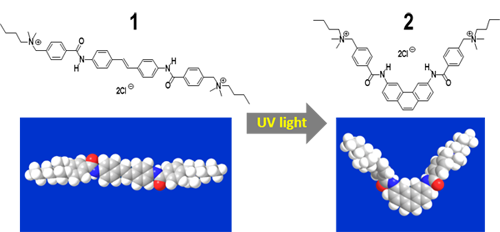
The researchers focused on an aromatic compound, stilbene, as a photoreactive group. Stilbene changes from E- to Z-form (photoisomerization) when subjected to UV irradiation and then it changes to phenanthrene through dehydrogenation using dissolved oxygen as the oxidant (Fig. 1). To achieve both SWCNT dispersion and photoreactivity, a photoreactive stilbene group was used as the core and benzamide groups with an ammonium group, that make the molecule water soluble, are introduced at the termini of the molecule based on molecular design. Indeed, a new soluble stilbene compound 1 (Fig. 2) was synthesized with a high yield through a two-step reaction using commercially available reagents, and the compound was evaluated.
 |
|
Figure 1 : Photoisomerization and cyclization reaction of stilbene |
 |
|
Figure 2 : Structural formula of the photoresponsive SWCNT dispersing agent (top) and the molecular model of the agent (bottom) |
The optimized structures obtained by molecular orbital calculation shows that the stilbene compound 1 has a linear and highly planar structure and that the molecule becomes V-shaped when the compound changes to a phenanthrene compound 2 due to UV irradiation (Fig. 2). Owing to the significant contribution of this change in the molecular structure to the difference in the interaction of the molecule of the dispersing agent with the SWCNT surface (its affinity to the SWCNT surface), adsorption (dispersion of SWCNTs) and desorption (aggregation of SWCNTs) can be controlled by UV light.
Figure 3 shows the evaluation of the change in the dispersion of SWCNTs due to the difference in the molecular structure of the dispersing agent. Due to its very high affinity to the SWCNT surface, the stilbene compound 1 was adsorbed on the surface and broke the strong intermolecular interaction between SWCNTs, dispersing them into water and allowing observation of the absorption spectrum characteristic of molecularly dispersed SWCNTs (black line in Fig. 3). Since the stilbene compound 1 was positively charged, the SWCNTs covered by the compound 1 were coated with an electrostatic layer having a high water affinity. This prevented aggregation of the SWCNTs by electrostatic repulsion and the SWCNTs were isolated and dispersed stably in water. This made the observation of the absorption spectrum possible. In contrast, the V-shaped phenanthrene compound 2 has a low affinity to the SWCNT surface and cannot be adsorbed on the SWCNT surface, making it unable to disperse the SWCNTs in water (blue line in Fig. 3).
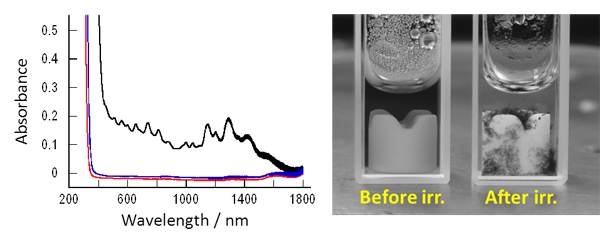 |
Figure 3 : Change in absorption spectra (in heavy water) due to the change in molecular structure (left)
and change in the dispersion of SWCNTs before and after UV irradiation (before irradiation: dispersion, after irradiation: aggregation) |
An SWCNT dispersion solution prepared using the stilbene compound 1 as the dispersing agent was continuously irradiated by UV light while being vigorously stirred. The SWCNTs were not able to remain dispersed in the water and aggregated (red line and the right photo in Fig. 3). Due to UV irradiation, the photoreaction (cyclization and oxidation) of the stilbene compound 1 on the SWCNT surface shown in Fig. 1 gradually proceeded and the stilbene compound 1 changed to a phenanthrene compound 2 with a low affinity to the SWCNT surface (currently, the required reaction time is about 6 hours). This is the first success in using the photocyclization of stilbene to control the dispersion state of SWCNTs. In a control test without UV irradiation, the SWCNTs did not aggregate, indicating that the SWCNT aggregation was due to UV irradiation. In this study, the evaluation of dispersion states was carried out using SWCNTs that can be evaluated easily by measuring the absorption spectra. The dispersion control technology could be easily applied to other types of CNTs.
This technology to control the dispersion of SWCNTs with UV light is novel. The researchers aim to improve the switching speed between dispersion and aggregation states and to develop photoresponsive dispersion agents that can be used repeatedly through new molecular design. They also aim to establish a mass synthesis technique, keeping the sample supply to external organizations in mind. In collaboration with other groups, the researchers will develop applications of this new technology in the fields, such as in advanced SWCNT purification and CNT device fabrication, where the cover of CNTs by dispersion agents often makes evaluation of their characteristics difficult.