Researchers) TOMINAGA Kenichi, assigned to the team, Functional Group Transformation Team, Interdisciplinary Research Center for Catalytic Chemistry
- AIST has developed a solid catalyst enabling to substitute CO2 for a reaction that uses highly toxic carbon monoxide as a raw material
- Continuous reaction using a flow reactor achieves ten times the hourly yield of batch reaction using the conventional tank reactor
- Contribute to the realization of carbon recycling
Continuous alcohol synthesis process using CO2 as a raw material
As a carbon-free society is advocated, technology for utilizing generated CO2 is demanded. In particular, new chemical synthesis processes that use CO2 as a direct raw material are expected in the chemical industry.
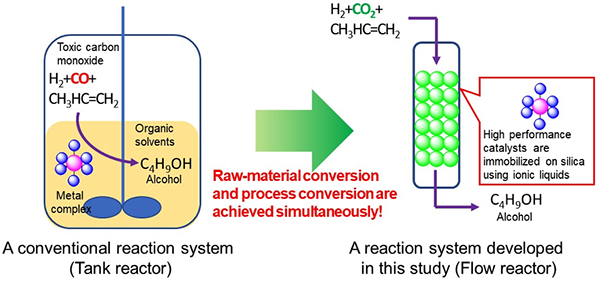
Hydroformylation reaction (oxo reaction) is one of the important processes in the petrochemical industry, and more than 10 million tons of alcohol and aldehydes are produced annually using these reactions. These reactions conventionally use propylene or other unsaturated hydrocarbons, carbon monoxide (CO), and hydrogen as raw materials, and are performed in batch reactors using metal complexes of cobalt and rhodium as catalysts.
Batch processes using this type of metal-complex catalyst have the issue that continuous production is not possible, and there are also issues with the post-reaction separation of catalysts and products and the reuse of catalysts. In order to address these issues, a number of methods have been proposed to immobilize metal complexes in solid carriers and use them like solid catalysts. However, there were issues such as changes in reactivity and low heat resistance compared to use of metal-complex catalysts alone.
Technology for continuous alcohol production using CO2 as a raw material was developed by AIST in collaboration with Hokkaido University.
In this technology, a metal-complex catalyst that functions to react CO2 and hydrogen with propylene to convert it into butanol, which is a type of alcohol, is immobilized on silica gel by an ionic liquid to make it a solid catalyst, which is known as SILP (supported ionic liquid phase) catalyst. By installing this solid catalyst in a flow reactor and performing the reaction, it was possible to produce butanol with CO2 continuously and at a 10-fold higher reaction rate.
Because butanol is one of the important products in chemical industry, this technology will promote carbon recycling. This result was published in ACS Sustainable Chemistry & Engineering, a journal of American Chemical Society (doi: 10.1021/acssuschemeng.1c02084).