Researchers: HIMEDA Yuichiro, Prime Senior Researcher, ONISHI Naoya, Senior Researcher, Carbon-based Energy Carrier Research Team, Global Zero Emission Research Center, KANEGA Ryoichi, Researcher, Energy Storage Group, Research Institute for Energy Conservation
A novel multinuclear catalyst was developed for highly selective hydrogenation of carbon dioxide to methanol by under the mild conditions of the lower temperature and pressure.
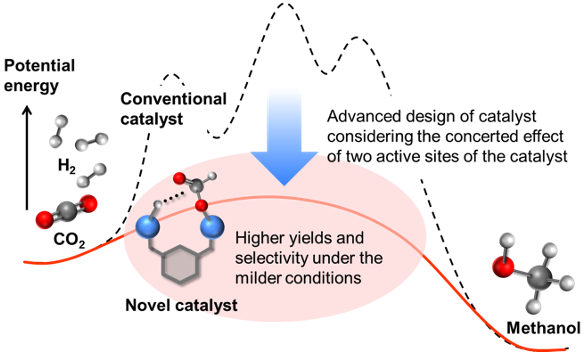
Production of methanol by the effective hydrogenation of carbon dioxide using a multinuclear iridium catalyst
The development of carbon recycling technology that converts carbon dioxide into useful chemicals is an urgent issue in order to achieve the government's new goal of reducing greenhouse gas emissions to virtually zero. Around 100 million tons of methanol are produced annually worldwide as a chemical raw material and alternative fuel, and various efforts have been made to develop a catalyst that converts carbon dioxide into methanol, one of the major industrial chemicals. However, the conventional copper-based solid catalysts require a reaction temperature of 200 °C or more. In addition to the formation of by-products such as carbon monoxide and methane, a low conversion rate was an issue due to equilibrium constraints between carbon dioxide and methanol at this reaction temperature range. Therefore, the typical technical theme is to lower the reaction temperature for methanol production from carbon dioxide with the higher conversion rate and selectivity.
The researchers at AIST developed a novel approach toward methanol production from CO2 using multinuclear catalyst including two iridium atoms in a gas-solid phase, in order to avoid the thermodynamic constraints. This catalysis promoted hydrogenation of CO2 to methanol even at 30 °C or 0.5 MPa with high selectivity. The achievements in this study will contribute to the zero emission of greenhouse gas in future.
The researchers aim to improve the catalyst performance, the total production efficiency, and cost reduction of the process.