Hiroaki Tateno (Senior Researcher) and Jun Hirabayashi (Prime Senior Researcher) of the Biotechnology Research Institute for Drug Discovery (BRIDD; Director: Masanao Oda), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), and Yasuko Onuma (Senior Researcher) and Yuzuru Ito (Leader) of Stem Cell Engineering Research Group, BRIDD, AIST, in collaboration with Life Science Research Laboratories, Development Headquarters I, Division of Laboratory Specialty Chemicals of Wako Pure Chemical Industries, Ltd. (President and CEO: Shinzo Kobatake), have developed a technology to eliminate human induced pluripotent stem cells and embryonic stem cells (hiPSCs/ESCs), which might be tumorigenic, from transplanting cells.
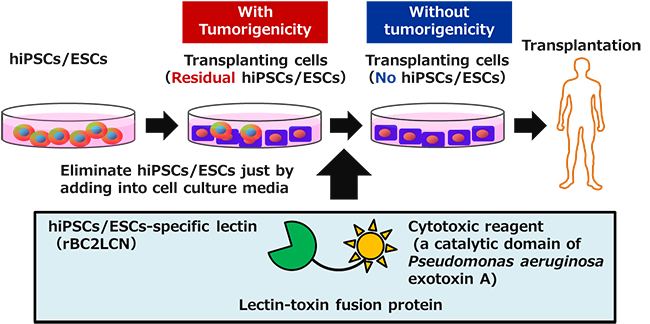
Transplanting cells, which are generated by differentiation of hiPSCs/ESCs, may contain residual hiPSCs/ESCs that did not differentiate. These hiPSCs/ESCs have the potential to be tumorigenic, which is a large barrier to applying the hiPSC/ESC-based techniques to regenerative medicine. The developed technology, however, can efficiently eliminate residual hiPSCs/ESCs in transplanting cells, and is therefore expected to improve the safety of regenerative medicine using transplanting cells generated from hiPSCs/ESCs.
Details of this technology were published online in an American scientific journal, Stem Cell Reports, on April 9, 2015 (US Eastern Time).
 |
|
Summary of the technology to eliminate potentially tumorigenic hiPSCs/ESCs from transplanting cells |
hiPSCs/ESCs have the potential to proliferate indefinitely (self-renewal) and to differentiate into any cell type, such as cardiomyocytes or neurons (pluripotency); therefore, society has high expectations that they will be a cell source for regenerative medicine that may completely cure various diseases. It is difficult, however, to differentiate all hiPSCs/ESCs into a target cell type, and in some cases, a certain number of undifferentiated hiPSCs/ESCs may remain. Because these cells might form tumors when transplanted into a patient, it is necessary to eliminate them before the transplantation. In the conventional technology commonly used for this purpose, transplanting cells are separated into single cells and then residual hiPSCs/ESCs are eliminated from them using special equipment called a cell sorter. However, this technology has some problems, e.g., it cannot be applied to a cell sheet, etc.; its processing speed is slow; and it may harm the survival of the transplanting cells. To resolve these issues, a safe, reliable, and easy technology to eliminate hiPSCs/ESCs remaining in transplanting cells is required.
AIST has developed lectin microarrays as a technology to enable the rapid and highly sensitive analysis of glycans that cover the cell surface in high density. In recent years, in particular, AIST has been working on the development of technologies for quality assessment and selection of stem cells by comprehensive analysis of the glycans on the hiPSC/ESC surface using lectin microarrays (AIST research result announced on June 22, 2011). As a result, AIST found that one of the lectins (carbohydrate-binding proteins), rBC2LCN, specifically binds to hiPSCs/ESCs, and in collaboration with Wako Pure Chemical Industries then developed a reagent that can stain live hiPSCs/ESCs using the binding ability of rBC2LCN. In addition, the analysis of the binding mechanism of rBC2LCN to hiPSCs/ESCs demonstrated that rBC2LCN binds to a specific O-glycan on a glycoprotein, podocalyxin (AIST press release on March 19, 2013). Subsequent research revealed that podocalyxin is secreted into the culture medium by various types of hiPSCs/ESCs. Based on this finding, AIST has developed a technology to easily detect hiPSCs/ESCs remaining in transplanting cells using cell culture medium (AIST research result announced on February 17, 2014).
This research and development was conducted as collaborative research between AIST and Wako Pure Chemical Industries (from FY2011) funded by the company.
In this research, it was found that rBC2LCN is internalized into hiPSCs/ESCs after binding to them. Based on this finding, the researchers developed a recombinant lectin-toxin fusion protein, in which the C-terminal of rBC2LCN is fused with Pseudomonas aeruginosa exotoxin, which induces cell death by inhibiting protein synthesis when internalized into cells.
Figure 1 shows microscopic images of human induced pluripotent stem cells (hiPSCs) 24 hours after being treated with the purified lectin-toxin fusion protein. The cytosol of live cells and the nuclei of dead cells are stained by a green fluorescent substance and a red one, respectively. When the culture medium did not contain the lectin-toxin fusion protein (0 µg/mL), many hiPSCs attached to the culture dish and were detectable with green fluorescence, but not red fluorescence, suggesting that most of the hiPSCs were alive.
On the other hand, when the culture medium contained 10 µg/mL lectin-toxin fusion protein, most cells lost the ability to attach to the culture dish and floated in the culture medium. Even a small number of cells that attached to the dish displayed red fluorescence, which indicates only dead cells. This result suggested that most of the hiPSCs were dead. The same effect was observed in human embryonic stem cells that were treated with the lectin-toxin fusion protein. These results demonstrated that the newly developed lectin-toxin fusion protein can efficiently eliminate various types of undifferentiated hiPSCs/ESCs.
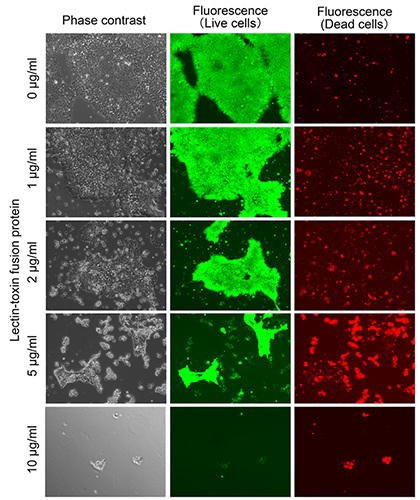 |
Figure 1: Effect of lectin-toxin fusion protein on hiPSCs
Phase contrast (left), green fluorescence (middle), and red fluorescence (right) show actual cell shapes, live cells, and dead cells, respectively. |
To investigate an effect on differentiated cells, the lectin-toxin fusion protein (10 µg/mL) was used to treat retinoic acid (RA)-differentiated hiPSCs, dermal fibroblasts, and adipose-derived mesenchymal stem cells. Almost all cells displayed green fluorescence, but not red fluorescence, indicating that the differentiated cells were alive (Fig. 2). This result suggested that the lectin-toxin fusion protein selectively eliminates undifferentiated hiPSCs/ESCs without affecting the proliferation and survival of differentiated somatic cells.
The developed lectin-toxin fusion protein can selectively eliminate undifferentiated hiPSCs/ESCs without affecting differentiated cells in culture by simple addition of the protein to culture medium without pretreatment of the cells, such as disassociation, and it can be used for large amounts of cells and cell sheets. It is expected to be utilized in various applications, including the generation of transplanting cells for regenerative medicine and cell preparation for drug discovery screening.
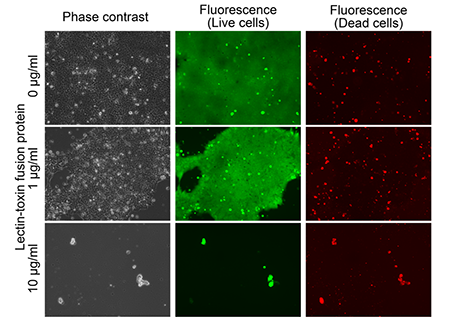 |
Figure 2: Effect of lectin-toxin fusion protein on differentiated cells
Phase contrast (left), green fluorescence (middle), and red fluorescence (right) show actual cell shapes, live cells, and dead cells, respectively. |
The lectin-toxin fusion protein is planned to be put into practical use within one year as a reagent to eliminate hiPSCs/ESCs. The researchers will contribute to improving the safety of regenerative medicine using transplanting cells generated from hiPSCs/ESCs by evaluating whether the fusion protein can be applied to cells for regenerative medicine, such as cardiomyocytes or neurons derived from hiPSCs.