Update(MM/DD/YYYY):05/09/2012
Opening the Way to a Redefinition of the Kilogram Based on Fundamental Physical Constants
- Successful improvement of the accuracy of the Avogadro constant -
Points
-
Measurement technology with one-nanometer accuracy for the shape of a silicon sphere by the accurate control of laser wavelength has been developed.
-
In an international project, an isotopically enriched silicon crystal was produced and the accuracy of the Avogadro constant has been upgraded.
-
Establishment of the mass standard not by an artificial object but by fundamental physical constants is becoming a reality.
Summary
The Metrology Institute of Japan (MIJ; Director: Yukinobu Miki) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi) has upgraded the accuracy of the Avogadro constant in an international research project in collaboration with the national metrology institutes of France, Italy, Australia, Germany, the United Kingdom, the United States, and the European Commission (the International Avogadro Coordination Project).
The Avogadro constant expresses the number of atoms or molecules contained in 1 mole of substance and is one of the important fundamental physical constants used in the fields of physics and chemistry. If the Avogadro constant is measured highly accurately, the unit of mass presently defined as the mass of the international prototype of the kilogram can be redefined based on the mass of an atom. In the International Avogadro Coordination Project, a crystal of enriched 28Si, one of the isotopes of silicon, was produced and the Avogadro constant has been determined by the x-ray crystal density method in which the density, lattice constant, and molar mass of the 28Si crystal were measured. AIST has measured the shape of 1-kg 28Si single crystal spheres with 1-nm accuracy by a laser interferometer equipped with a newly developed system that tunes the wavelength of laser, and determined the volume and density of the spheres. The Avogadro constant has been determined with accuracy of one digit better then ever before (3×10-8) by combining the lattice constant, molar mass, and density obtained in the international research cooperation. This result was published in a US scientific journal, Physical Review Letters (2011, volume 106, page 030801).
Taking into account the upgrade of the accuracy of the Avogadro constant by AIST and others, an agreement on the future abolition of the international prototype of the kilogram and the implementation of redefining the kilogram based on fundamental physical constants has been reached at the General Conference of Weights and Measures (CGPM) held in October 2011. Establishment of a mass standard that does not depend on artificial objects is becoming a reality for the first time in history.
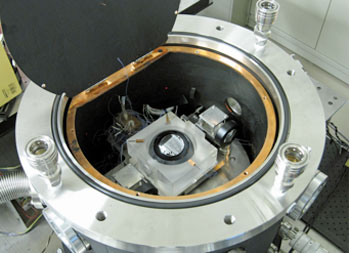 |
Photo 1 : Laser interferometer developed by AIST that measures the shape of the silicon spheres
with 1-nm accuracy by accurate control of the wavelength of laser |
Social Background of Research
The International System of Units is the universal system of units composed of seven base units (m, kg, s, A, K, cd, mol) of length, mass, time, electricity, thermodynamic temperature, luminous intensity, and amount of substance and derived units. Its origin goes back to the Convention of the Metre concluded in 1875 which became a cornerstone of modern weights and measures. At the memorable first General Conference of Weights and Measures, the international prototype of the meter and the international prototype of the kilogram made of platinum-iridium alloy were approved as the unit of length and mass respectively. Later, redefinitions of units were carried out along with development and expansion of them. There have been two major redefinitions of the meter, the unit of length so far. Shifting to the definition based on the wavelength of light emitted by 86Kr and abolition of the international prototype of the meter in 1960, and also shifting to the definition using the speed of light in a vacuum in 1983 were the two redefinitions.
Meanwhile, the international prototype of the kilogram being only one in the world is used as the standard in the definition of the kilogram for more than 120 years since the adoption of the prototype. The international prototype of the kilogram is kept at the International Bureau for Weights and Measures (BIPM) in a Paris suburb, and mass standards in the world are maintained and managed through the chains of comparison with the national prototype of the kilogram of each country that was regularly calibrated with the international prototype of the kilogram. However, long term stability of the mass of the international prototype of the kilogram is estimated to be about 50 µg due to surface contamination and so on. Although this corresponds to slight fluctuation of 5×10-8 against 1 kg, it is becoming not negligible due to the progress of measurement technology in recent years. Shifting to the definition of the kilogram based on fundamental physical constants like that of the meter has been discussed at the International Committee on Weights and Measures (CIPM).
Two ideas of the definition of the kilogram based on fundamental physical constants are now proposed; one definition is based on the Avogadro constant that determines the mass from the number of atom and the other is based on the Planck constant that links the mass and the photon energy by the photoelectric effect and the theory of relativity. Therefore, the determination of these two constants more accurately than the long term stability of the international prototype of the kilogram (5×10-8) has been aspired for the redefinition of the kilogram.
It is considered that there is no direct effect from the redefinition of the kilogram by fundamental physical constants in our daily lives. However, the redefinition of the meter by the speed of light made it possible to measure the exact length in the order of nanometers by lasers, and it has established the foundation of nanotechnology that controls a substance at the atomic level. Realization of an exact mass standard based on fundamental physical constants also has a possibility to bring a breakthrough and innovation to cutting edge science and industrial technology including nanotechnology through the fundamental technology for exact mass measurement at the atomic level.
History of Research
AIST launched an accurate measurement of the Avogadro constant by using silicon about 40 years ago. Silicon has an advantage of its well-known physical properties due to the semiconductor research conducted so far. Furthermore, it is relatively easy to prepare a defect-free, large, highly pure single crystal of silicon. Although the mole is currently defined by the carbon atom with mass number 12 (12C), the Avogadro constant, the number of atoms or molecules contained in 1 mole of a substance, can be determined highly accurately using a silicon crystal if the mass ratio of 12C and silicon atom is measured accurately.
At first, an experiment to measure the lattice constant of a silicon crystal was started. In 1987, a technique to polish a silicon crystal to a sphere close to an exact sphere was developed, and that made it possible to measure the density of a silicon crystal highly accurately. AIST developed a laser interferometer that can measure the shape of a 1-kg silicon sphere polished with ultra-precision of sphericity of several tens of nanometers. In 1994, the volume of the silicon sphere was measured in a vacuum for the first time in the world and the world's highest accuracy of the density of solids was achieved by measuring its volume without the effect of the refractive index of air.
It is necessary to measure the silicon isotopic ratio accurately in order to determine the molar mass of silicon (average atomic weight) because of the existence of three stable isotopes, namely 28Si, 29Si, and 30Si. In 2003, AIST measured the molar mass of silicon in collaboration with the Institute for Reference Materials and Measurements (IRMM; Director: Krzysztof Maruszewski) of the European Commission and determined the Avogadro constant with the best accuracy of that time, 2×10-7 (AIST press release on January 20, 2004). Nevertheless, further improvement in accuracy could not be expected due to the difficulty of accurate measurement of the molar mass.
In order to resolve this problem, AIST started the International Avogadro Coordination Project in cooperation with 7 metrology institutes in 2004 to determine the Avogadro constant using a silicon single crystal in which only 28Si was enriched. Besides AIST, BIPM (Director: Michael Kühne), Istituto Nazionale di Ricerca Metrologica (INRIM; Director: Alberto Carpinteri) of Italy, IRMM, National Measurement Institute (NMIA; Director: Laurie Besley) of Australia, National Physical Laboratory (NPL; Director: Brian Bowsher) of UK, National Institute of Standard and Technology (NIST; Director: Patrick Gallagher) of US, and Physikalisch-Technische Bundesanstalt (PTB; Director: Joachim Ullrich) of Germany participated in the International Avogadro Coordination Project and carried out the project in the field that each institute was specialized in.
Details of Research
In the International Avogadro Coordination Project, it took two years to obtain 99.99% isotope-enriched silicon tetrafluoride (28SiF4) using a centrifuge technique at Russia. By using that 28SiF4 as raw material, a 28Si single crystal of 5 kg was prepared in 2007. From this crystal, two spheres of 94-mm diameter, 7-nm sphericity, and 1-kg mass were polished. In order to determine the density of these spheres, AIST has developed a new laser interferometer that measures the shape of silicon sphere with accuracy of 1 nm through the accurate control of the laser wavelength (Photo 1). For the accurate measurement of the shape of sphere, the interferometer is installed in a vacuum chamber equipped with a system that measures and controls a temperature of the silicon sphere with accuracy better than 0.001 °C.
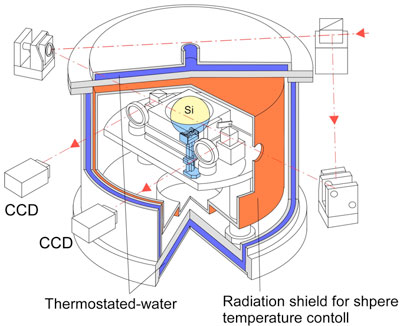 |
|
Figure 1 : Vacuum chamber that houses the laser interferometer for measuring the shape of the silicon sphere |
It is equipped with the system that measures and controls the temperature of the sphere with accuracy
better than 0.001 °C to accurately measure the sphere shape. |
Moreover, for the accurate measurement of the volume, AIST has developed a surface analysis method by combining a x-ray reflectivity technique and spectroscopic ellipsometry and measured the thickness of the oxide layer on the surface of the sphere accurately (Photo 2). The density of the sphere was determined from the mass and the volume corrected for the effect of the surface oxide layer. The homogeneity of lattice constant in the crystal was verified by using a facility of High Energy Accelerator Research Organization as the evaluation of the crystal. Finally, the Avogadro constant has been determined with an accuracy of 3.0×10-8, one order of magnitude better accuracy than before, from the density, the lattice constant, and the molar mass measured by the research institutions participating in the project, including AIST. The International Avogadro Coordination Project published the result in a US scientific journal. The determined Avogadro constant has been adopted as data for the fundamental physical constants adjustment of the Committee on Data for Science and Technology (CODATA) which was published in June 2011.
 |
Photo2 : Spectroscopic ellipsometer used for the surface analysis of the 28Si-enriched spheres (left)
and film thickness measuring instrument by the x-ray reflectivity technique (right) |
Sphere surface analysis traceable to the national metrology standards is possible using the spectroscopic ellipsometer calibrated by a reference material.
The reference material is calibrated by the film thickness measuring instrument using the x-ray reflectivity technique; the instrument is used to certify
the thin film reference materials provided by AIST. |
In 2007, NIST directly measured the Planck constant by the watt balance method from the measurements of voltage and electrical resistance determined by the Josephson effect and the quantum Hall effect and determined the constant with an accuracy of 3.6×10-8 . By fundamental upgrading of the accuracy of the Avogadro constant by the International Avogadro Coordination Project, the accuracy of both the Avogadro constant and the Planck constant is better than 5×10-8. Taking this situation into account, the resolution approving future abolishment of the international prototype of the kilogram and redefining the kilogram based on fundamental physical constantats was adopted at the General Conference on Weights and Measures, the highest decision-making body of the Convention of the Metre, held in October 2011. The establishment of a mass standard not by artificial objects but by fundamental physical constants such as the Avogadro constant and the Planck constant is becoming a reality for the first time in history.
In the discussion concerning the redefinition of the kilogram at the General Conference on Weights and Measures, the Avogadro constant obtained using the silicon crystal was compared with the Avogadro constant derived from the Planck constant that was obtained from the watt balance method (Fig. 2). Although the value measured by the International Avogadro Coordination Project agrees with the value measured by NPL and Bundesamt für Metrologie (METAS) of Switzerland within the uncertainty. However, it does not agree with the value measured by NIST, the most accurate value determined by the watt balance method, and differs in seventh digit. This discrepancy was the major reason why the kilogram was not redefined at the conference in October 2011. International research collaborations are planned in order to identify the cause of this discrepancy by improving the accuracy of each method.
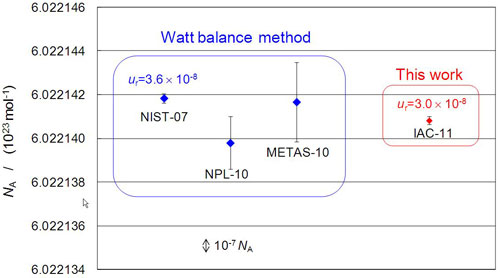 |
|
Figure 2 : Comparison of the values of the Avogadro constant determined by different measurement principles |
Bars on each data represent the standard uncertainties of experimental data.
NIST-07: derived from the Planck constant by the watt balance method, NIST in 2007,
NPL-10:derived from the Planck constant by the watt balance method, NPL in 2010,
METAS-11:derived from the Planck constant by the watt balance method, METAS in 2011,
IAC-11: measured by the International Avogadro Coordination Project in 2011 |
Future Plans
Concerning the Avogadro constant determined using the silicon crystal, five research institutes, namely AIST, BIPM, INRIM, NMIA, and PTB, will start new international research collaboration in 2012. More accurate measurement of the Avogadro constant is expected through the improvement of the volume measurement of the 28Si-enriched silicon spheres. As for the Planck constant obtained by the watt balance method, measurements are being carried out at Laboratoire National de Metrologie et D’essais of France and National Research Council Canada as well as at NIST and METAS. It is planned to identify the cause of the discrepancy between the x-ray crystal density method and the watt balance method based on these independent high-accuracy experiments.