Update(MM/DD/YYYY):08/22/2011
Simulation of phenomena from optical excitation to charge splitting in organic materials for solar cells
- Analyzing the microscopic photovoltaic phenomenon in a charge-transfer complex -
Points
-
Computational technique to numerically calculate dynamics of electron orbitals in molecules
-
Visible light can induce the photovoltaic phenomenon because of the difference between electron affinities of two molecules.
-
Potential contribution to the development of solar cells using organic charge-transfer complexes
Summary
Yoshiyuki Miyamoto (Leader), Dynamic Process Simulation Group, the Nanosystem Research Institute (Director: Kiyoshi Yase) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), has performed the first-principles simulations that consistently treat from optical excitation to electron-hole splitting in a photovoltaic material formed by molecules having different electron affinities in collaboration with Dr. Mina Yoon, Oak Ridge National Laboratory, USA, and Prof. Matthias Scheffler, Fritz Haber Institute, Germany.
This technology numerically solves the time-dependent Schrödinger equation to simulate electron orbitals that change with time by light irradiation, hence enables us to perform microscopic computation of entire photovoltaic processes from optical excitation to charge splitting consistently. By applying this technique to designing molecular structures of charge-transfer complexes, contribution to the improvement in energy conversion efficiency of organic solar cells is expected.
The details of the research will be published in New Journal of Physics, a scientific journal published by the German Physical Society and the British Institute of Physics, on July 28, 2011 (British local time).
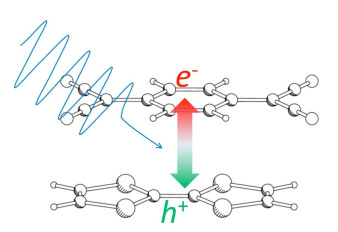 |
|
(Figure) Schematic of photo-excited electron (e-) and hole (h+) in molecules that will soon move to opposite directions
|
Social Background of Research
Due to the recent sever situation in energy supply, high reliability and safety as well as low cost and high efficiency are required for energy supply technology. Solar cell technology has a long history as a technology for clean, safe, and renewable energy. Organic solar cells have emerged in this decade with their advantages such as light-weight, flexibility, and low production cost. Yet, organic solar cells are required further improvement in photoelectric energy conversion efficiency for practical applications. Moreover, organic materials have wide variety of structures compared to silicon based photovoltaic materials. Therefore, correct understanding of photoelectric energy conversion mechanisms and molecular design of organic photovoltaic materials based on the understanding are necessary in order to effectively improve the efficiency of organic solar cells. Although simulation technology is indispensable for molecular design, simulations of indivisual properties are not enough. The efficiency of photoelectric energy conversion is governed by a wavelength range of usable light, yield of photo-excitation, and electron-hole splitting rate, and these parameters correlate with each other. Therefore, a new simulation technique that can treat all of these parameters consistently is required for speedy achievement of high efficiency of organic solar cells.
History of Research
At AIST, the researchers have been developed a computational technology called the first-principles simulation which is an elemental technology of material (molecular) design. Recently, they are using time-dependent first-principles simulation code to monitor many kinds of phenomena in materials that are triggered by electronic excitations, and were aware that such code is also useful to study the photovoltaic phenomenon. Then this code is used to examine a potential photovoltaic material that consists of a famous donor molecule (TTF) and an acceptor molecule (TCNQ) by performing a simulation.
This work was supported by “HPCI Strategic Program, Field 2, New Materials/Energy Harvesting” (FY2011-FY2015) commissioned by the Ministry of Education, Culture, Sports, Science and Technology. All calculations were performed by using the T2K Supercomputer system at the University of Tsukuba.
Details of Research
TTF and TCNQ molecules have properties of donor and acceptor of electrons, respectively. The molecular complex consisting of a TTF molecule and a TCNQ molecule is expected to form a minimum unit of a pn-junction that shows a photovoltaic phenomenon. If the complex shows a photovoltaic phenomenon, a macroscopic system made of the minimum units should work as a solar cell. However, it is impossible to measure the photovoltaic phenomenon of such a small unit experimentally. Therefore, the photovoltaic phenomenon of this unit was examined by performing the time-dependent first-principles simulation. TTF-TCNQ complex might have a stable structure in which molecular planes are parallel and molecular axes are in the same direction. Intermolecular distance in this structure is 0.3 nm. (Fig. 1)
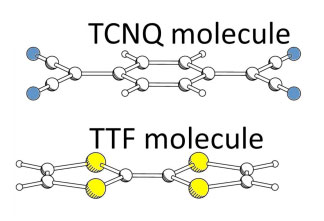 |
Figure 1 : Molecular complex of TCNQ which tends to accept electrons and TTF which tends to provide electrons
Blue circles are nitrogen atoms, yellow circles are sulfur atoms,
open circles are carbon atoms, and small open circles are hydrogen atoms. |
By applying the time-dependent first-principles code, an absorption spectrum was calculated as shown in Fig. 2. The spectrum shows peaks at photon energy of 2 eV (Reddish orange light, wavelength 620 nm) and at 3.5 eV (ultraviolet light, wavelength 354 nm). The simulation also predicted that the absorption of light polarized parallel to the molecular axis is strong.
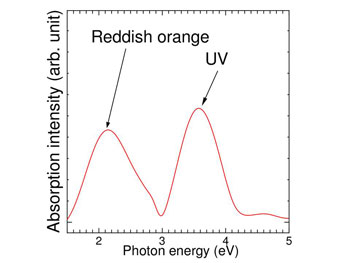 |
Figure 2 : Simulated absorption spectrum
Strong absorption peaks are seen for reddish orange and UV lights. |
In this molecular complex (charge-transfer complex), there is electron transfer from the TTF molecule to the TCNQ molecule causing polarization. In order to simulate how this polarization is modified by light irradiation, time-dependent modulation of the dipole moment (charge distribution) was calculated by applying an alternating electric field with the frequencies corresponding to the wavelengths (frequencies) of irradiated lights. The computed result shows the dipole moment increases with light of photon energy of 2 eV (reddish orange color) as schematically illustrated in Fig. 3. By connecting this molecular complex with electrodes, the increased dipole moment contributes to the photovoltaic phenomenon.
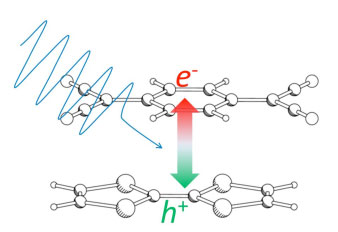 |
|
Figure 3 : Schematic of increase in transfer of electron and hole caused by light irradiation
|
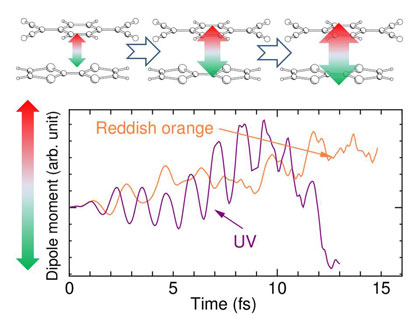
Figure 4 shows time-dependence of the dipole moment induced by alternating electric fields (light irradiation) corresponding to reddish and ultraviolet (UV) lights. The dipole moment increased with the light of photon energy 3.5 eV (UV) as in the case of reddish light, but the UV light could not sustain the increase of the dipole moment. Hence it is concluded that visible reddish light can induce the photovoltaic phenomenon with high efficiency and that invisible UV light cannot induce the phenomenon. Major components of sunlight are visible and near infrared lights, and UV light is relatively weak. Therefore, this molecular complex, in which the photoelectric energy conversion efficiency by visible light is higher than that by UV light, demonstrates its potential for application as a solar cell.
 |
|
Figure 4 : Schematic of increasing electron-hole splitting as a function of the irradiation time (top)
and calculated time-dependence of the dipole moment (bottom)
The electron-hole splitting caused by reddish orange light is larger than that induced by UV light, suggesting a higher efficiency.
|
Future Plans
The solar cell using a charge-transfer complex, which requires no electrolyte, is expected to have longer lifetime than that of a dye-sensitized solar cell, while the photovoltaic efficiency is relatively low. It is expected that further improvement in the efficiency by designing materials of organic solar cells is achieved by using this simulation technology.
For further improvement in efficiency, simulations that can give information about lifetime of organic solar cells will be important. The researchers therefore plan to develop simulation technology that can examine molecular structure change and destruction throughout the photovoltaic phenomenon, and can evaluate the toughness of molecules against UV light irradiation which tends to degrade organic molecules. Meantime, they also plan to investigate influence of the relative position of donor and acceptor molecules as well as the efficiency of inter-molecular transport and loss by recombination of photo-excited carriers (electrons and holes) by numerical calculations.