- It is operable at a practical low temperature: application to exhaust gas-treatment devices is expected -
Koichi Hamamoto (Research Scientist), the Functional Assembly Technology Group (Group Leader: Masanobu Awano), the Advanced Manufacturing Research Institute (Director: Hideto Mitome) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) developed a new electrochemical reactor that decomposes and purifies NOx in diesel engine exhaust gas at a low temperature with high efficiency.
The electrochemical reactor with nano-structured electrodes, decomposes NOx in diesel engine exhaust gas containing high concentrations (about 20%) of oxygen at a lower temperature than 250℃. Furthermore, it improves the fuel efficiency because of the reduction in energy needed to purify exhaust gas. This technology enables the construction of a system that combines air quality conservation with a reduction in CO2 emission. From our point of view, such systems should substitute traditional catalytic systems for exhaust purification in light of the prospective tightening of legal restrictions.
The research results will be exhibited at HANNOVER MESSE 2008, held from April 21 to 25, 2008, in Hannover, Germany.
|
|
|
Fig. Schematic view of an electrochemical reactor with nano-structured electrodes
|
An improvement in the efficiency of and a reduction in the environmental load imposed by vehicles are earnestly required to prevent the global warming and conserve the environment. Furthermore, legal restrictions are tightening worldwide with regard to reducing CO2 emission by improving fuel efficiency, and reducing the emission of environmental pollutants such as NOx.
Highly-efficient engines, such as lean-burn engines and common-rail diesel engines, have been studied extensively with the aim of improving their fuel efficiency. However, the amount of NOx emission from these highly-efficient engines is increased due to the increase in combustion temperature and pressure. Furthermore, conventional three-way catalysts cannot decompose and purify NOx fully because of the high concentration of oxygen in exhaust gas. It has proved difficult to combine an improvement in fuel efficiency with the purification of exhaust gas. Recently, as a promising purification technology under high concentrations of oxygen, selective catalytic reduction catalysts with NOx adsorbability are under development. However, they have many problems including the additional consumption of fuel used as a reducing agent, and the collateral emission of harmful substances resulting from the use of ammonia, because they are currently hard to be controlled precisely. The establishment of an innovative NOx purification technology that meets the tightening legal restrictions on exhaust gas is essential to put highly-efficient engines into practical use, and the achievement of such a technology is hoped for strongly.
In Japan, particularly, there is a high proportion of cars driving at a slow speed, or traveling for a short distance in urban areas. Next-generation diesel engines with a idling stop function are now under development. For these reasons, technologies that efficiently purify NOx from vehicle exhaust gas at a low temperature range are desired.
AIST successfully developed in 2001 an electrochemical reactor with a solid electrolyte that used an electrochemical reaction as a new purification system for exhaust gas. The reactor was able to decompose and remove NOx with high selectively under conditions of the coexistence of oxygen. However, it was hard to put the reactor to practical use because of the difficulty in lowering its operating temperature. We have devised electrodes having a new structure, based on an analysis of the highly-selective decomposition mechanism of NOx, and have developed a highly-efficient NOx decomposition reactor that operates at a low temperature.
On the working electrode (cathode) of an electrochemical reactor, both the NOx in exhaust gas and the coexisting oxygen decompose simultaneously at the same reaction site, in general. If decomposition at the reaction sites is not highly NOx selective, most of the electric power is expended in decomposing the oxygen that coexists in exhaust gas at a concentration more than 1,000 times larger than that of NOx. Namely, oxygen molecules (O2) are ionized at a cathode, and oxygen ions (O2-) move through an electrolyte and are emitted as oxygen molecules at an anode (ion pumping).
We replaced the electrolyte of the existing NOx decomposition reactors, developed by our group, with materials that have high oxygen ion conductivity for the purpose of achieving operation at a low temperature. The replaced reactors were able to work at a low temperature, but their decomposition selectivity with regard to NOx dropped off drastically, and the coexisting oxygen was decomposed dominantly.
In this research, we used nano-structured working electrodes, while using an electrolyte that has high oxygen ion conductivity. Due to the nano-structured electrodes, reactivity and amount of reaction sites, i.e. a three-phase boundary of electrode, electrolyte and gas phase, were increased. An atmosphere in which NOx decomposed dominantly was also realized at the electrodes. As a result, the new reactor can be operated at a much lower temperature than the previous one.
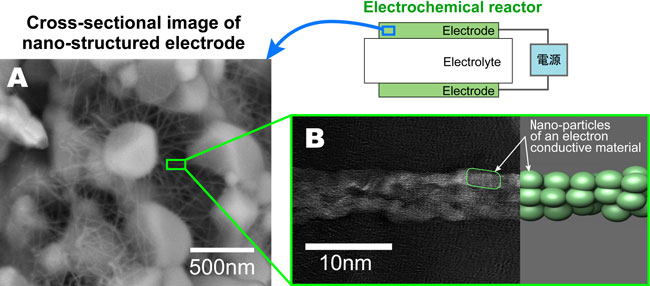
In the newly developed electrochemical reactor, gadolinia-doped ceria (GDC) that has a high oxygen ion conductivity was used as a solid electrolyte substrate, and working electrode layers that decomposed NOx selectively were formed on both sides of the substrate using a screen-printing method. The working electrode layers were composite of GDC and electron-conductive materials. Through electrochemical treatment, a three-dimensional nano-network structure was formed in the layers, where fine fiborus electron conductive materials 10 nm in diameter were wound around a framework made of connected GDC particles about 500 nm in diameter, as shown in Figure 1A. The fine fiborus electron conductive materials were composed of assembled nano-particles having a diameter of a few nm as shown in Figure 1B. This rugged fiber structure enabled a large increase in the area of the three-phase boundary, that is, the reaction sites of NOx decomposition, while electron conducting paths were secured.
An electrochemical reactor fitted with the above-described nano-structured electrodes showed high reactivity to NOx and was able to decompose about 90% of NO gas of 1,000 ppm at the low temperature of 250℃ in an atmosphere consisting of 20% of oxygen and 80% of nitrogen (see Figure 2). The oxygen concentration of this atmosphere was higher than the actual oxygen concentration of diesel engine exhaust gas. It is almost impossible for conventional catalysts to decompose NOx under such condition.
As shown in Figure 3, this new electrochemical reactor can operate at a lower temperature of 250℃ in an oxygen-rich atmosphere without performance degradation, in comparison with the old AIST electrochemical reactor, which had the world's best purification efficiency at the time. For that reason, this technology is a promising purification technology for exhaust gas emitted from next-generation diesel engines.
 |
|
Fig. 1 A: Cross-sectional SEM image of nano-structured electrode. B: High-resolution TEM image and schematic illustration of fine fiborus electron conductive materials
|
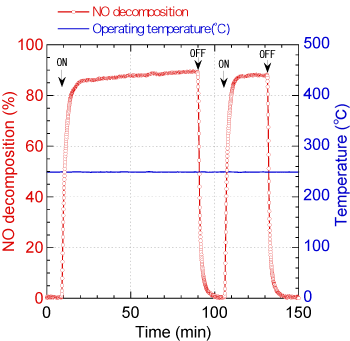
Fig. 2 NO decomposition property under high oxygen concentration condition (20%) at 250℃
|
|
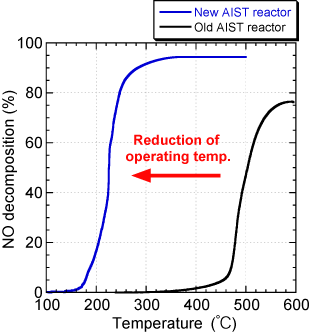
Fig. 3 Operating temperature dependence on NO decomposition property of the electrochemical reactors.
|
Aiming for practical use, we will pursue a lower operating temperature and denser reaction sites, evaluate the durability of the reactor and the influence of coexisting gases, and make improvements as necessary. We also want to realize a integrated electrochemical exhaust gas purification system by integrating this research with a high-sensitive rapid-response NOx sensor and the technology of a device that purifies NOx and particulate matter (PM) simultaneously, both of which have been already developed by AIST. We also want to establish a technology contribute to the solution of energy and environmental issues.