The National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi) signed a memorandum of understanding for comprehensive research cooperation (MOU) with the Pacific Northwest National Laboratory (PNNL; Director: Steven Ashby) under the U.S. Department of Energy.
PNNL is a national research institution located in the state of Washington and engages in research in a variety of fields including renewable energy, environmental health and remediation, computing research, biological sciences, and national security. The Environmental Molecular Sciences Laboratory (EMSL), which is one of PNNL’s user facilities, is equipped with many sophisticated precision instruments and a supercomputer, and scientists come to use the facility from all over the world.
AIST’s Department of Energy and Environment and Department of Materials and Chemistry have been promoting cooperation with PNNL as part of the project commissioned from the Ministry of Economy, Trade and Industry (METI) called, “International Joint Research and Development Project on Innovative Energy Technologies” (hereinunder “Innovative Project”). The notable R&D project includes the development of a hydrogen carrier system that enables safe storage, transport, and production of hydrogen through interconversion between carbon dioxide and formic acid.
Under this MOU, AIST aims to further encourage collaborative research and personnel exchange with PNNL and to enhance AIST’s presence as an international research hub by working to further strengthen its international R&D capabilities and industrial competitiveness.
 |
|
Pacific Northwest National Laboratory (PNNL) |
In order to efficiently utilize renewable energy, it is strongly encouraged to develop technologies to transform primary energy to hydrogen as clean secondary energy (forms of energy that are processed and transformed from the primary energy obtained from nature) and then to use it as an energy carrier (a hydrogen storage substance) that is easy to transport over long distances and able to store energy in a large scale. As an energy carrier, it must meet various requirements; it needs to be liquid at room temperature, to possess high hydrogen storage density, to be converted with low energy when storing or releasing hydrogen, to be low in cost, and to be easily provided in high volume as a storage medium.
Against this background, AIST has focused on formic acid as an energy carrier which is liquid at room temperature and has a high storage density of hydrogen. In 2007, PNNL and AIST started on joint research on hydrogen production. This cooperation, in 2014, led to the success in the development of a novel catalyst with the world’s highest efficiency among heterogeneous catalysts, which is essential for the practical application of a hydrogen storage system utilizing formic acid. Moreover, AIST developed the technology to continuously provide high-pressure hydrogen from formic acid without the use of a compressor. This new technology made possible energy conservation and low-cost operation in compression process during the production of high-pressure hydrogen, which had been deemed difficult. (AIST press release on December 11, 2015, titled “Development of Continuous High-Pressure Hydrogen Supply Method without any Compressor”) This technology will accelerate the realization of a hydrogen economy.
|
Image of High-Pressure Hydrogen Evolution Reaction |
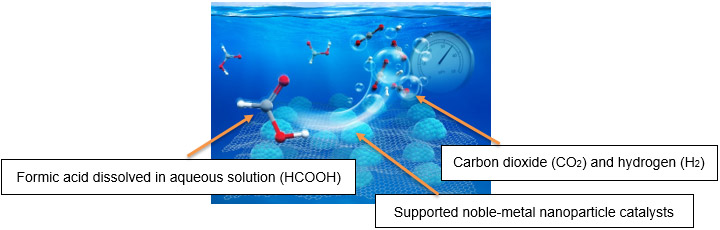 |
Currently, four AIST research units, namely the Research Institute of Energy Frontier, the Research Institute of Electrochemical Energy, the Renewable Energy Research Center, and the Research Institute for Chemical Process Technology, are collaborating with PNNL, and they are working to further advance the research outcomes they have so far achieved and are proceeding to develop technologies for hydrogen storage and utilization using carbon dioxide under the Innovative Project. From 2016, they are also working to develop a metal hydride for hydrogen storage to establish highly-efficient, low-cost technologies for hydrogen storage and utilization under the said project.
PNNL has an ultrahigh-resolution Nuclear Magnetic Resonance (NMR) instrument installed at EMSL, which is advantageous for the analysis of catalytic reaction. This instrument enables in situ analysis of catalytic states and chemical reactions under high pressure conditions, which is the one and only instrument in North America. AIST is currently developing new materials and reaction processes based on the data acquired through the use of this state-of-the-art equipment.
 |
|
Ultrahigh-resolution NMR instrument which enables in situ analysis of catalytic states (Photograph courtesy of PNNL) |
Considering the long-standing research collaboration between PNNL and AIST as described above, this MOU was concluded to further solidify the cooperative relationship. AIST anticipates accelerating the research collaboration as part of the Innovative Project as well as facilitating researcher exchange. PNNL and AIST will further promote organizational cooperation and work toward the realization of a hydrogen economy.
Pacific Northwest National Laboratory
The Pacific Northwest National Laboratory (PNNL) is one of the laboratories of the U.S. Department of Energy (DOE). The Battelle Memorial Institute has been a contractor of DOE to manage and operate PNNL since 1965. As of FY 2016, 4,485 staff are employed at PNNL, and the annual budget amounts to US$ 920 million, of which 73% is from DOE; 20% is from other federal, state, and regional governments; and one percent is from private entities. PNNL has its main campus in Richland, WA, and the satellite offices in Seattle, WA; Portland, OR; and Washington D.C. PNNL’s areas of research cover a broad range including renewable energy, environmental remediation, computing research, biology, and national security. There are two user facilities at PNNL: the Environmental Molecular Sciences Laboratory (EMSL) and the Applied Process Engineering Laboratory (APEL). EMSL is designed for molecular-level research in physics, chemistry, and biology to find solutions for environmental challenges. APEL is available for scientists and engineers from companies, public organizations, and universities as a place where they can demonstrate their new technologies, and test their prototypes and pilot plants. The number of PNNL’s patents issued in FY 2016 is 104, and the total number of domestic or foreign patents acquired since 1965 is 2,514.