Masaki Torimura (Leader) and Sung-Bae Kim (Senior Researcher), Measurement Technology Group, the Research Institute for Environmental Management Technology (Director: Hiroaki Tao) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), created superluminescent luciferases with artificial design, exerting greatly prolonged bioluminescence. AIST has studied luciferases derived from luminous plankton (copepods) motivated by their small molecular weight and strong luminescence. In the present study, the researchers aligned the amino-acid sequences of a variety of copepod luciferases and identified frequently occurring amino acids, which were further rearranged on the basis of their unique idea (a multiple sequence alignment rule inside the made sequence), and finally developed a series of unique luciferases that are genetically distinctive from natural luciferases. Considering that the made luciferases are distinctive from luciferases derived from natural organisms, they were named artificial luciferases (ALuc). ALuc are up to 100 times brighter than conventional luciferases and exhibit excellent luminescence sustainability (half-life: 20 minutes).
The made ALuc were examined in a variety of assay systems making use of conventional luciferases (reporter-gene assay, two-hybrid assay, and bioimaging of living organisms). The results revealed that ALuc is advantageous over any conventional luciferases in terms of enhancement in sensitivity, reduction of the measurement time, and light permeability in the tissues of living organisms. The present development of ALuc as a luminescent marker will contribute to basic research in life science and is further expected to contribute to a variety of diagnoses that have been limited by problems of poor sensitivity and measurement speed: e.g., high throughput screening of biomarkers in the field of medical diagnoses in hospitals, personal healthcare in households, and high-sensitivity analysis of endocrine disrupting chemicals in water and food in the field of environmental diagnoses.
Part of this research was conducted in an AIST internal project, "Asia Strategy—Water Project" (FY2012-2013). Details of this technology will be published online in a US scientific journal, Bioconjugate Chemistry, in the near future.
 |
|
The developed artificial luciferases (ALuc) |
As the general public's understanding on bioluminescence has emerged since Dr. Osamu Shimomura was awarded the Nobel Prize in chemistry in 2008, the use of bioluminescence in fields of life science, medical diagnosis, and environmental surveillance has been growing under the public consensus.
Luminescence of biomolecules can be roughly divided into luminescence resulting from excitation by external light energy (fluorescence) and luminescence resulting from excitation by the energy from a chemical reaction (chemiluminescence). Measurement of fluorescence has limitations in terms of compactness and assay simplicity because it requires an excitation light source, optical filters, etc. In the case of bioluminescence as a typical kind of chemiluminescence, luciferases (such as luciferase derived from fireflies) are used as a catalytic means for converting chemical energy into light. However, the industrial application of these luciferases has been limited because of the poor features in brightness and luminescence sustainability.
In contrast to the wide use as chemiluminescent labels, the luciferases derived from fireflies (firefly luciferase, or FLuc) and from sea pansies (Renilla luciferase, or RLuc) still have problems in signal reliability and long-time measurement, which are mainly caused by weakness and instability of emission signals. Conventional copepod luciferases (GLuc and MpLuc1) also have the same problems as emission stability and brightness. To address these problems, the researchers attempted to create artificial luciferases carrying optimal amino acids, instead of the direct use of native luciferases derived from luminous organisms.
As many copepod luciferases were recently discovered by AIST researchers, the sequences were multiply aligned to identify the most frequently occurring amino acids. The found consensus amino acids were utilized in creating a series of artificial luciferases. Some of the created luciferases were found to exert great optical intensity and stability.
Since 2011, noting the potential industrial value of luminescence in labeling, AIST has conducted basic researches on the optical properties of luciferases from marine animals and their luminescence mechanisms. In the present study, the researchers first reviewed characteristics of the amino-acid sequences of conventional luciferases obtained from copepods and reanalyzed their functionality.
It is believed that more frequently occurring amino acids are the consequence survived by natural selection over a long period of evolution and thus would contribute to thermodynamic stability of the whole sequence. Upon alignment of the amino-acid sequences of more than 10 kinds of copepod luciferases, the researchers could make thermodynamically stable whole amino-acid sequences from the regions filled with frequently occurring amino acids. Further, the researchers found that the copepod luciferases consist of two consensus domains that are responsible for the catalytic reaction of the enzymes. The researchers also found that the increased homogeneity of the consensus domains improves the optical performance (brightness and stability).
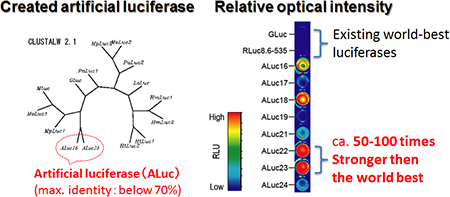
On the basis of the above findings, the researchers created a series of artificial luciferases (ALucs) with a linkage of the extracted key amino acids, which were significantly distinctive from existing luciferases. The amino-acid sequences of ALuc greatly differ from those of conventional luciferases and the sequential identity was generally less than 70 %, compared to existing luciferases (Fig. 1 left). In addition, each of the made luciferases was found to have its own characteristics in terms of optical brightness, emission wavelength, luminescence sustainability, substrate specificity, heat resistance, extracellular secretion, etc. The various optical properties of the luciferases enabled the researchers to generate assay systems optimal for the diverse assay purposes and environments. In particular, the enzymes with excellent optical intensity and sustainability were especially useful for enhancing sensitivity and throughput in diagnoses.
The advantages of the made ALuc as a luminescent marker were examined in the following methods:
(1) When the luciferase genes are introduced into an animal cell and expressed equivalently, ALuc are up to approximately 100 times brighter (Fig. 1 right) and up to 7 times more stable than the brightest among the conventional luciferases (GLuc and RLuc8.6-535).
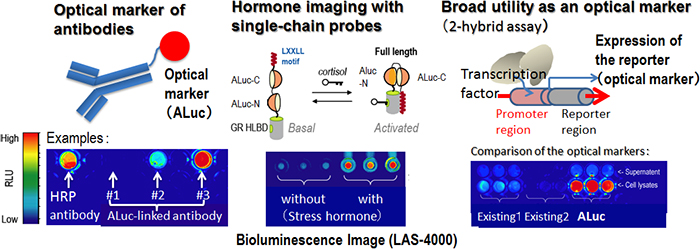
(2) An antibody was made and labeled with the present luciferase to measure a disease marker (antigen) in medical diagnoses. This ALuc-labeled antibody was generated and compared the optical intensities with those of a conventional HRP-labeled antibody. The ALuc-labeled antibody (type 3) was found to be approximately 2 times brighter than the HRP-labeled one (Fig. 2 (A)). Further, ALuc emitted longer wavelength bioluminescence than HRP did, which exerts better tissue permeability (bioimaging) than shorter one.
(3) To estimate human stress levels, the researchers developed a single-chain luminescent probe comprising ALuc and the ligand binding domain of stress hormone (cortisol) receptor. The probe exhibited brighter bioluminescence than the probe using GLuc and thus made it possible to measure the stress hormone even in human saliva (Fig. 2(B)).
(4) Two-hybrid assays are useful for the measurement of the transcription activity of chemical substances (environmental assay) and the determination of protein-protein interactions (life science). When transcription activity of a chemical substance or protein-protein interactions of interest occur, the reporter gene is designed to be expressed, and thus the optical intensities of the expressed reporter quantitatively represent the concentration of the chemical substance or the impact of the protein-protein interactions. ALuc produced greater brightness than GLuc did under the same conditions (Fig. 2(C)).
 |
|
Figure 1 : Comparison of artificial luciferases (ALuc) and conventional high brightness luciferases |
 |
|
Figure 2 : Application of ALuc to luminescent labeling in bioassays
(A) Example of ALuc used as an antibody label. Types 1, 2, and 3 are luminescent antibodies produced using different antibody production methods.
(B) Operating principle of single-chain-type bioluminescent probe. ALuc is divided in two and temporarily deactivated. The stress hormone causes the divided ALuc to fold into its original form, which in turn restores the luminescence activity of the ALuc.
(C) Example of a two-hybrid assay in which ALuc is used for luminescent labeling. When the transcription factor is activated, a reporter is expressed. |
The present development of ALuc raises the expectation of other new high-performance enzymes in the future, not merely discovering natural enzymes. The future goals related to luciferase development include the determination of the three-dimensional structure of luciferases, creation of more red-shifted bioluminescence for the better optical permeability in the tissues of living subjects, and the development of mass production methods. Meanwhile, the present examples promise the further emerging usage of the luciferases in the field of diagnosis through further adjustments of the experimental conditions including modification of the amino acids inspired by the analysis of the three-dimensional structures of ALucs. For the emerging use, the research team will advance joint research with companies that desire optical marker sales corresponding to the industrial needs. In addition, the researchers desire to provide advanced luciferase technologies that are convenient to a wide range of individuals in households, not just at medical care and environmental measurement facilities, by making light-detecting devices smaller and more portable as the supporting technology, and by developing testing kits and test papers with the ALuc technologies.