Masaru Yoshida (Leader) and Haruhisa Akiyama (Senior Researcher), Smart Materials Group, the Nanosystem Research Institute (Director: Kiyoshi Yase) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have developed materials that are able to be repeatedly liquefied and solidified at constant room temperature by light irradiation alone.
Each of the new photo-reactive materials is a liquid crystalline substance produced by the combination of a skeleton of sugar alcohol and azobenzene groups, and is able to be repeatedly liquefied and solidified by irradiation of wavelength-controlled light without heating or cooling. This phenomenon is the first example of a selective and reversible transition between solid and liquid states of a single substance through the action of light alone at room temperature. The use of these materials is expected to contribute to the development of entirely new highly functional materials such as reusable and reworkable light-controlled adhesives.
Details of this technology will be published in a German scientific journal, Advanced Materials, on May 2, 2012.
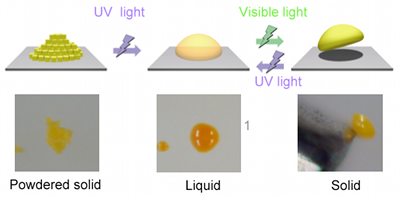 |
|
With UV light irradiation, the solid powder liquefied and forms droplets; visible light irradiation resolidifies the material. |
A diverse range of organic materials are widely used in information devices, home appliances, transportation equipment, etc. Further performance improvements, such as more sophisticated functions and reduced weight, are expected for these materials. Generally, these organic materials are solidified from a liquid state when formed. Heating and melting the raw materials is the method normally employed in these operations, but solidification by drying following dissolution in a solvent or solidification through chemical bonding of liquid raw materials are also used in forming. The technologies for reversible and precise control of basic property changes such as phase transition from liquid to solid and from solid to liquid are expected from the perspective of enabling the conservation of resources and energy, which are important factors in the realization of sustainable development. However, to date there have been no single substance able to be repeatedly changed between liquid and solid states at room temperature by light irradiation alone, without heating or cooling.
AIST has been actively conducting research on photo-reactive organic materials. Up to the present, it has developed a material that can be melted from a crystalline state at room temperature by light irradiation and returned to its original solid state by heating (AIST press release on December 2, 2010). The researchers pursued research seeking to enable a reversible physical change between liquid and solid states at room temperature to be controlled using light alone. The researchers discovered that reversible liquefaction and solidification by light was possible in multi-branched compounds, each of which was formed by introducing a large number of photo-reactive azobenzene groups to the molecule having a skeleton of an easily obtainable sugar alcohol.
This research and development project was supported by Grants-in-Aid for Scientific Research (C) (FY2009 - FY2011) of the Japan Society for the Promotion of Science.
The developed materials are multi-branched compounds. AIST has previously developed them as liquid crystalline materials with multiple photo-reactive groups in a single molecule (paper published in August 2009), which are now used as additives in color display and recording materials that can be rewritten using light while remaining color-fast.
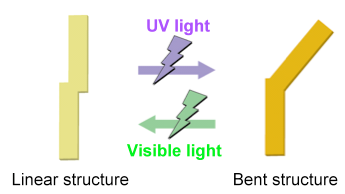
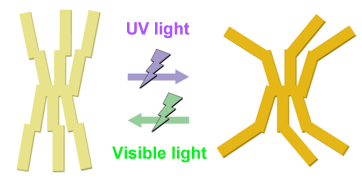
The developed materials employ azobenzene groups as their photo-reactive moieties. Azobenzene is known to change reversibly between a rod-like structure and a bent structure (isomerization) by light irradiation (Fig. 1). However, in its crystalline state, it is rare for azobenzene to undergo this isomerization, because its crystallinity is high and therefore the free-volume space that is necessary for structural change is small. The materials also feature a structure in which the terminal parts of the multiple azobenzene groups are densely connected at the center (Fig. 2, left). The materials are synthesized as powder (solid), but are known to change into a liquid crystalline state at high temperatures when heat treated. This liquid crystalline state is thought to be induced because the densely connected parts at the centers of the molecules hinder the uniform arrangement of the azobenzene groups that tend to crystallize. At room temperature lower than the temperature of the liquid crystalline state, the materials are thought to be in a liquid crystalline glassy solid state or a non-uniform crystal-like solid state, which have lower crystallinity than that of crystalline states, due to the same effect of inhibition of the uniform molecular arrangement. Even in the solid state, therefore, the materials are expected to possess a certain amount of free-volume space, i.e. photo-reactivity.
Photo-reactivity of the materials was examined and the materials were found to possess good isomerization reactivity even in the solid state. Specifically, when the yellow powder, the raw material, was irradiated by UV light (LED light source; Center wavelength: 365 nm; Intensity: 40 mW cm-2), the material changed in color to orange as the isomerization reaction proceeded and at the same time gradually liquefied, finally reaching a completely liquid state. When this liquid was irradiated by visible light (LED light source; Center wavelength: 510 nm; Intensity: 20 mW cm-2), the material returned to its original yellow color and solidified as the azobenzene groups isomerized and the molecules returned to their original rod-shaped structure. It was possible to repeat these photo-liquefaction and photo-solidification reactions.
|
 |
|
Figure 1 : Structural changes in azobenzene by light irradiation |
Figure 2 : Structural changes in a developed compound
with six azobenzene-substituted groups by light irradiation |
Easily available sugar alcohols are used as the basic skeletons of the photo-reactive materials. Multiple photo-reactive azobenzene groups are ester bonded to the skeletons to synthesize the materials. Because the synthesis of the materials is extremely simple and raw materials are readily available, it is suited to large-scale synthesis. Threitol, D-mannitol, xylitol dimer, and other substances with multiple hydroxyl groups were used as the sugar alcohol. For comparison, derivatives of methanol and ethylene glycol, which have fewer hydroxyl groups, were also synthesized. Compounds with one, two, four, six, and eight azobenzene substitution groups were synthesized and compared. It was found that no photo-reactions occurred in compounds with one or two substitution groups and that photo-liquefaction occurred in compounds with four or more substitution groups.
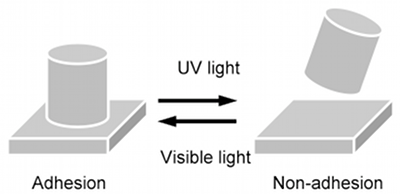
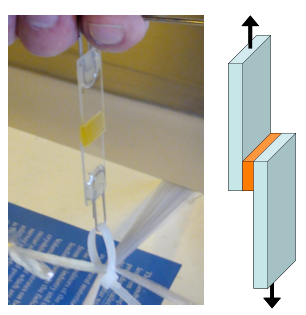
These new photo-reactive materials can be expected to have a variety of applications. One potential application is in photo-reactive adhesives enabling repeated application and removal (Fig. 3). Change in adhesive force due to light irradiation was tested (Fig. 4). Tensile shear strength was measured using a simple method in which the liquefied material was sandwiched between two glass plates and a weight was suspended from the glass after the solidification of the material. A figure of 50 N cm-2 was obtained (at a film thickness of approximately 6 µm). Following this, UV light was irradiated through the glass plate in order to liquefy the material. The tensile shear strength was measured again and found to be less than 0.3 N cm-2. As this indicates, UV light irradiation resulted in a significant decline, to almost zero, in adhesive strength. The liquid was next irradiated by visible light and resolidified and tensile shear strength was again measured. Shear strength had returned to the initial figure of 50 N cm-2, demonstrating that the material recovered its adhesive performance through visible light irradiation.
 |
|
Figure 3 : Photo-controlled adhesion and non-adhesion |
 |
|
Figure 4 : Simple adhesion performance test |
The major characteristic of the developed materials is the fact that it is possible to control their liquefaction and solidification through the irradiation of light of different wavelengths alone under normal conditions (room temperature). The researchers aim to develop various applications of the materials in areas suited to their new characteristics including adhesion control by light. In addition, because the synthesis of the materials is suited to mass production, the researchers would provide test specimens to external organizations and conduct joint research projects. At the same time, they will go on seeking and developing new photo-reactive materials that have improved performance.