-The materials reversibly become pink by blue light and colorless by green light –
Morito Akiyama (Leader) of Process Measurement Team, Hiroshi Yamada (Senior Researcher) of Advanced Integrated Sensing Team, and Kazufumi Sakai (Invited Senior Researcher) of Optical Measurement Solution Team, the Measurement Solution Research Center (Director: Hisatoshi Hirai) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have developed composite metal oxide materials that show stable and reversible photochromism when irradiated alternately with blue and green monochromatic lights.
The materials were prepared by adding metallic elements to barium-magnesium silicate (BaMgSiO4) under reductive atmosphere. The color density of the materials can be controlled by the wavelength of irradiation light; for example, the materials turn light pink when they are irradiated with laser light of 405 nm (blue) and deep pink when they are irradiated with ultraviolet light of 365 nm. They become colorless again when they are irradiated with laser light of 532 nm (green). These color changes are reversible. The changes in the color of the materials when irradiated with light of different wavelengths remain almost the same even after the materials are repeatedly irradiated more than ten times, indicating the excellent durability of the materials. In the future, the materials are expected to have applications including ultrahigh-density optical memories, rewritable paper, and displays.
The results of this study will be published in Journal of the Ceramic Society of Japan, a scientific journal of the Ceramic Society of Japan.
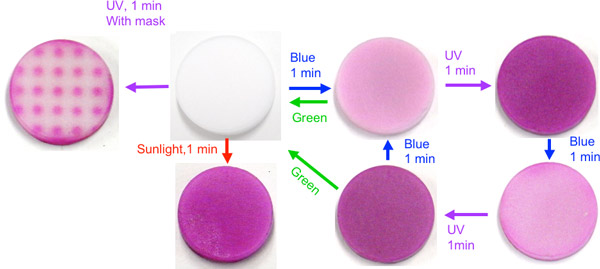 |
|
Figure 1 Photochromic properties of iron-added BaMgSiO4 |
For a long time, inorganic photochromic materials have been expected as a type of materials for high-density memories and displays. In particular, there have been extensive studies on inorganic photochromic materials responsive to visible light that can be used to develop compact ultrahigh-density memories using a semiconductor laser or LED as the light source. However, most inorganic materials showed no photochromism responsive to visible light, had low durability, or showed slow color change. They also posed the problem of poor reversibility, i.e. failed to decolorize after being irradiated several times. In addition, the color of most of these materials was blue.
AIST has conducted research and development to improve the visible-light response and durability of conventional inorganic photochromic materials. We explored materials that showed photochromism when irradiated with blue and green lights, so that semiconductor lasers and LEDs could be used as light sources. Further, we investigated into the effects of the preparation conditions and addition of metallic elements into the materials, and other factors in order to improve the photochromic properties of the materials.
In this study, we mainly explored fluorescent composite metal oxides and found that barium-magnesium silicate (BaMgSiO4) prepared under reductive atmosphere showed photochromism responsive to visible light. Figure 2 shows the crystal structure of BaMgSiO4 and the X-ray diffraction pattern of the BaMgSiO4 material prepared in this study.
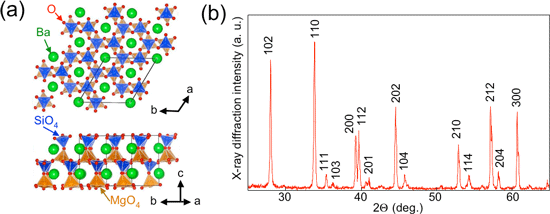 |
|
Figure 2 (a) Crystal structure of BaMgSiO4; (b) X-ray diffraction pattern of BaMgSiO4 (Digits indicate the diffraction planes.) |
BaMgSiO4 has a tridymite structure, and the SiO4 tetrahedrons are connected at the corners to form three-dimensional tunnels (Fig. 2a). Half of Si4+ ions are replaced with Mg2+ ions, and the Ba2+ ions are embedded in the tunnels. We investigated the crystal structure of the prepared sample by X-ray diffraction and found that all the X-ray diffraction peaks matched the peak pattern corresponding to the tridymite structure. Therefore, it was confirmed that the tridymite structure is retained even when the material is prepared under reductive atmosphere (Fig. 2b).
Figure 3 shows the reflection spectra and photochromic properties of BaMgSiO4 prepared under argon atmosphere (BMS) and of BaMgSiO4 prepared under argon atmosphere containing 5% hydrogen, i.e. reductive atmosphere, (BMS-H). No decrease is observed in the visible-light reflectance for both samples before they are irradiated (Fig. 3a). However, we found that the reflectance of BMS-H at 523 nm decreased when the sample was irradiated with blue light (405 nm), and its color changed to light pink (Fig. 3b). Further, when BMS-H was irradiated with green light (532 nm), following the blue light irradiation, it became colorless again and reversible photochromism was confirmed. Furthermore, when BMS-H was irradiated with ultraviolet light (365 nm), reflectance of a wide visible light wavelength range centered at 523 nm reduced, while BMS showed only a slight reduction in the reflectance at around 523 nm (Fig. 3c). The color of BMS-H at this point was deep pink, and it was also confirmed that the material becomes colorless again when it was irradiated with green light after the ultraviolet light irradiation. It is interesting to note that the reflection spectrum did not change (the color did not become deeper) when we continued to irradiate the material with blue light, and that the color changed from deep pink to light pink when the material was irradiated with blue light after irradiation with ultraviolet light. Thus, we found that the density of the color varied depending on the irradiation light.
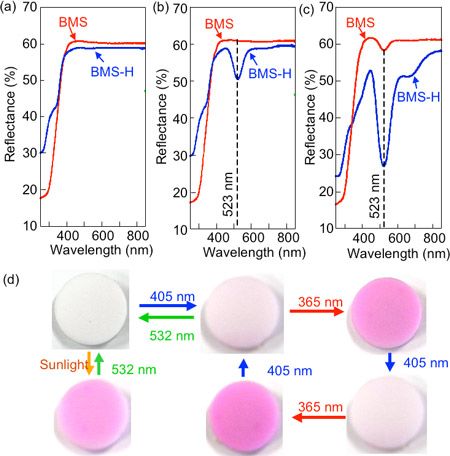 |
|
Figure 3 (a) Reflection spectra before irradiation, (b) reflection spectra after irradiation with blue light (405 nm), (c) reflection spectra after irradiation with ultraviolet light (365 nm), and (d) photochromic properties of BaMgSiO4 |
The effects of addition of various metallic elements to BaMgSiO4 are examined, in order to improve the photochromic properties of BaMgSiO4 (Fig. 4). The reflectance at 523 nm dropped considerably when iron (Fe) or europium (Eu) was added. Repeated irradiation (more than ten times) has little effect on the color changes, indicating the excellent durability of the materials. Since Fe is far more readily available than Eu, one of rare earth elements, we expect the material containing Fe to have a wide range of applications in the future.
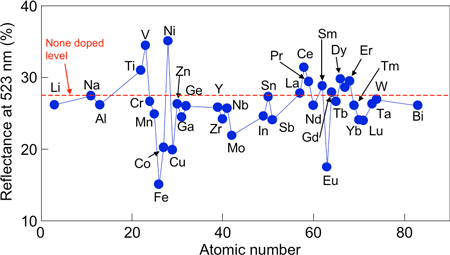 |
|
Figure 4 Effects of metallic element addition to BaMgSiO4 on photochromism when irradiated with ultraviolet light (365 nm) |
The mechanism of the photochromic phenomenon exhibited by the visible light responsive BaMgSiO4 materials is considered to be related to oxygen defects in the materials, since the phenomenon is observed in the materials prepared under reductive atmosphere. Namely, the materials absorb 523 nm light as the electrons excited by light irradiation are trapped by the oxygen defects, and BaMgSiO4 materials turn pink. We assume that the electrons trapped by the defects are excited again and return to their original state when the materials are irradiated with green light, and they lose their color. We also speculate that the color density changes with the wavelength of light because the transition probability of the electrons depends on the wavelength of the excitation light.
In the future, we plan to prepare thin films of BaMgSiO4 with added metallic elements in order to investigate their possibilities as materials for fabricating ultrahigh-density memories and displays.