Tsutomu Nakamura (Senior Research Scientist), the Functional Biomolecule Research Group (Leader: Jun Miyake), the Research Institute for Cell Engineering (Director: Jun Miyake) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Hiroyuki Yoshikawa) in collaboration with the group headed by Prof. Tsuyoshi Inoue of the Graduate School of Engineering, Osaka University analyzed and discovered a new mechanism of a primitive microorganism's protein reaction that removes active oxygen. Unlike previously known mechanisms, this mechanism has been confirmed to involve a hypervalent compound as a key player in the mechanism. Hypervalent compounds had not been found in natural substances so far.
This discovery concerns a new oxidation mechanism of a protein, which is anticipated to be applied in oxidative stress-related medical technologies, and which will contribute to the development of new methods in synthetic organic chemistry.
The research results were presented in the Proceedings of the National Academy of Sciences of the United States of America on April 29 , 2008 (EDT, U.S.A.).
 |
|
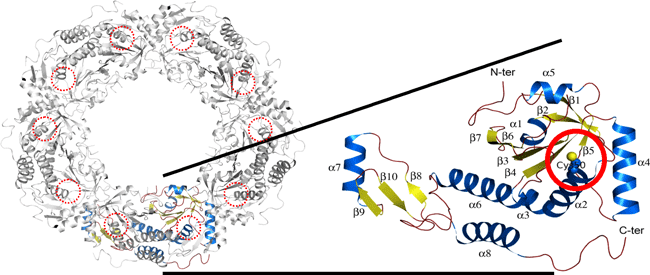
Figure The tertiary structure of the antioxidant protein, peroxiredoxin from Aeropyrum pernix .Red circles indicate the cysteine residues which are oxidized by hydrogen peroxide.
|
Oxygen is essential for life-sustaining activities such as breathing; however, as a result of exposure to ultraviolet radiation or side reaction of aerobic respiration, oxygen can turn into active oxygen with strong oxidizing effect. Damage caused by the active oxygen to cells is called "oxidative stress," which is believed to be related to diseases such as cancer, diabetes, arteriosclerosis and Alzheimer's disease, and to aging. In order to develop a medical technology that provides defense against the oxidative stress, it is important to learn how living organisms actually defend themselves, as in case of other medical technology. Living organisms have evolved to produce various antioxidant proteins so that they can defend their cells against the oxidative stress created by active oxygen. How these antioxidant proteins have evolved and what mechanism they use to remove active oxygen are fundamental and essential themes in the advancement of medical technology.
When life on the Earth began, oxygen was not accumulated in the Earth's atmosphere; however, as oxygen accumulated in the atmosphere, a mechanism to overcome the damage caused by active oxygen became indispensable for survival. Antioxidant proteins from the archaea are very useful materials in studying how, and by what mechanism, the physiological functions of the primordial life operated when it acquired the physiological functions enabling removal of active oxygen. This is because genetic research has suggested that a group of organisms, classified as the archaea, are considered to be the closest living relatives to the last common ancestor of all life.
AIST has been studying proteins from hyperthermophilic archaea growing at temperatures above 90℃ with the aim of utilizing thermostable proteins for industrial applications. Archaea are generally anaerobic, but there are some exceptions. Some archaea are aerobic, and it is anticipated that they may have a defense system to protect themselves from the oxidative stress caused by active oxygen. Aeropyrum pernix is one such aerobic hyperthermophilic archaea, the antioxidant system of which has been analyzed at AIST in order to get close to the earliest antioxidant system on the Earth. Meanwhile, at Osaka University, structural analyses of proteins have been conducted for the development of pharmaceuticals.
Based on these research activities at the two institutions, we elucidated the tertiary structure of a protein that removes hydrogen peroxide, an active oxygen species, with a focus on stereochemical changes associated with the removal reaction (oxidation of the protein).
This study was supported by "Crystallization Research," "Metabolic Systems" of "National Project on Proteins Structural and Functional Analyses" of the Ministry of Education, Culture, Sports, Science and Technology (FY 2005-2006) and the Grant-in-Aid for Scientific Research of Priority Areas "Structural Organization and Functional Mechanism of Biological Macromolecular Assemblies" from the Ministry of Education, Culture, Sports, Science and Technology (FY 2006-2007). Regarding the X-ray source for crystalline structural analyses, we used "SPring-8", the large synchrotron radiation facility, under the approval of a research project run by the Japan Synchrotron Radiation Research Institute.
Structural changes in antioxidant protein peroxiredoxin (Prx, see Figure 1) from an archaeon, Aeropyrum pernix, which removes hydrogen peroxide (an active oxygen species), was studied by means of X-ray crystallography and quantum chemical calculations. The removal of hydrogen peroxide by Prx proceeds as hydrogen peroxide oxidizes the cysteine residue (indicated by the red circle in Figure1 (c)) of Prx.
 |
|
Figure 1 The tertiary structure of the antioxidant protein, peroxiredoxin from Aeropyrum pernix
|
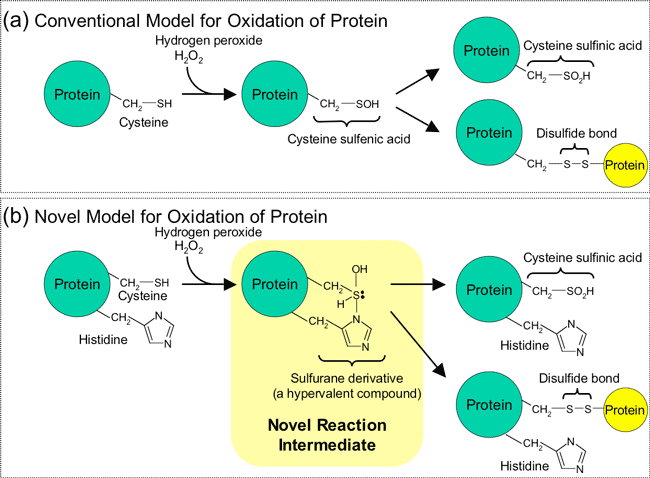
In conventional mechanisms, oxidation of cysteine residues of a protein is thought to occur through the formation of a cysteine sulfenic acid as an intermediate (Figure2 (a)). In Prx, however, oxidation reaction occurs through a novel mechanism in which cysteine and neighboring histidine residues form a sulfurane derivative that acts as a reaction intermediate (Figure2 (b)). This was confirmed by X-ray crystal structure analysis of the reaction intermediate in which a molecular structure corresponding to the sulfurane derivative was observed (Figure 3).
Sulfurane is a kind of hypervalent compounds. Hypervalent compounds are useful and have versatile applications in the field of reaction/synthetic chemistry. All the hypervalent compounds observed so far have been synthetic, and there have been no observations of hypervalent compounds with natural origin. This study shows for the first time that hypervalent compounds, which had been recognized and used only as synthetic substances, are actually utilized by living organisms as well.
Usually, reaction intermediates are unstable, the structure of which is very rarely determined by X-ray crystallography. In the current study, the key to the successful determination of the intermediate structure was the use of a unique method, i.e. after the protein crystal was first immersed in and pulled out from a solution containing hydrogen peroxide, cold nitrogen gas (at approximately -190℃) was used to freeze the protein crystal. The crystal was subjected to X-ray stractural analysis using synchrotron radiation of SPring-8. Unlike chemical reactions in solution, chemical reactions occurring within crystals can be quenched easily by removing the crystals from the solution, which helps prevent the reactions from proceeding too far. Moreover, we are able to fix the unstable reaction intermediate by immediately freezing the crystals after quenching the reaction, and thus measure the structure of the intermediate.
 |
|
Figure 2 Reaction mechanism of oxidation of cysteine side chains of proteins
Panel (a) shows the conventional model, in which a cysteine residue is oxidized to cysteine sulfenic acid. In this intermediate, the sulfur atom contains eight valence electrons. Panel (b) is the newly found reaction mechanism, in which the cysteine residue, together with a neighboring histidine residue, is oxidized to a sulfurane derivative, a hypervalent sulfur intermediate. The sulfur atom of the intermediate contains ten valence electrons. Both of the intermediates proceed either of the two pathways, one to the overoxidation to cysteine sulfinic acid or the other to the formation of disulfide bond. The intermolecular disulfide bonds are represented here, although the disulfide bond can occur within the same molecule.
|
This discovery concerns the elucidation of a new oxidation mechanism of active oxygen removal by an antioxidant protein, and add knowledge to the development of a new medical technology to counter the oxidative stress. Oxidative reactions of proteins are found not only in the removal of active oxygen, but also in many phenomena in living organisms. This discovery also proposes a new model for such general phenomena.
 |
|
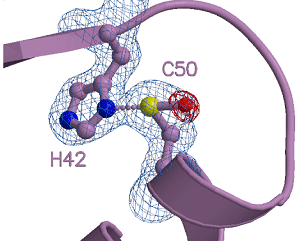
Figure 3 The tertiary structure of the novel reaction intermediate, a sulfurane derivative
Shown is the three-dimensional structure of the oxidation intermediate determined by X-ray crystallography. Sulfur atom of the cysteine (C50) residue and nitrogen atom of the histidine (H42) residue are connected with a covalent bond. Balls represent the atoms as follows: purple, carbon; blue, nitrogen; yellow, sulfur; and red, oxygen.
|
Regarding the synthesis of hypervalent compounds, the reaction pathway (oxidation of sulfur through a sulfurane derivative intermediate) elucidated in this study will provide hints useful to the development of sulfur-containing pharmaceuticals and industrial organic chemistry. This study also demonstrates that it is possible to "Learn from primordial life," even in the field of organic chemistry.
The reaction of the protein from the aerobic hyperthermophilic archaeon was analyzed in order to elucidate the primitive antioxidative system created in the very early period when oxygen started to accumulate in the Earth's atmosphere. It is interesting to note that a new mechanism of removing active oxygen was discovered in such a primitive system.
It is anticipated that the new mechanism of active oxygen removal will be applied in medical technologies targeting oxidative stress, canceration, and aging. Furthermore, this discovery would contribute to the development of new synthetic methods by offering a new method to oxidize sulfur atoms. We intend to conduct further studies aiming medical and industrial applications.