- Enabling the application of stabilized functional organic molecules to industrial materials -
The National Institute of Advanced Industrial Science and Technology (AIST; Hiroyuki Yoshikawa, President) has succeeded in the suppression of light-degradation of a photo-functional organic molecule, β-carotene, by encapsulating it in single-wall carbon nanotubes (SWCNT).
Organic materials have been expected to be applied to various areas such as electroluminescence (EL) displays. However, as they are more degradable than inorganic materials, improving their durability has been a big problem. For, example, various linear π-conjugated molecules, such as β-carotene, known as the coloring of carrots, have large third-order optical nonlinearity, and thus they have been expected to be one candidate for next-generation optical device materials. However, they have a problem which is light-degradation in air.
The AIST has found that β-carotene can be stabilized by encapsulation within SWCNTs. By the finding that large organic molecules like β-carotene (approximately 3 nm: 1 nm = 10-9 m) can be encapsulated inside CNTs at a temperature of approximately 70°C, which is fairly close to room temperature, hereafter the utilization of CNTs as nano-containers for improving the durability of organic molecules may be expected.
 |
|
 |
|
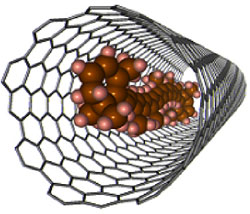
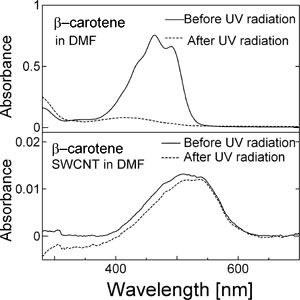
Figure 1 A carbon nanotube encapsulating β-carotene (left), and change in absorption spectra of the radiated ultraviolet light (right). For β-carotene alone (upper right), the peaks characteristic of β-carotene disappear, and for β-carotene encapsulated in a carbon nanotube (lower right) the peaks show only a very small change. |
As seen from the cases of liquid crystal- and organic EL-displays, the utilization of organic materials has been growing. However, organic molecules with special functions are usually degradable, and thus enhancement of durability has been one of the most important subjects in device fabrication.
Linear π-conjugated molecules are substances with large third-order optical nonlinearity and ultra-fast responsiveness, and thus their application to next-generation device, such as optical switching device, has been expected. However, because they are degradable by active species such as light-induced radicals, they can barely be used as optical device materials.
It is already known that fullerene (C60) and organic molecules can be encapsulated in CNTs. The encapsulated organic molecules are considered to be protected from outer active species (radicals etc.) by the CNT wall. Thus, we considered that light-degradation of π-conjugated molecules could be suppressed by encapsulation in CNT. Up to now, to encapsulate organic molecules within CNTs, a sublimation method has been predominantly used; in this method, first, the organic molecules and CNTs are sealed in glass tubes, and then they are heated with decreasing pressure using a vacuum pump to sublime the molecules, resulting in the encapsulation of the gas-state molecules in the CNTs. However, functional organic molecules are usually large, and can be degraded and decomposed by heating before sublimation. Thus, techniques enabling the encapsulation of large organic molecules inside CNTs have been desired at low temperatures.
The AIST has developed techniques to precisely control the diameter of SWCNTs and to encapsulate organic molecules in SWCNTs for nonlinear optical devices in the "Development of Carbon Nanotube Materials for Nonlinear Optical Devices" project for the Promotion of Industrial Technology and Research of the New Energy and Industrial Technology Development Organization. We previously observed a jet of water molecules from the inside of CNTs (nano-jet), and based on this observation, we considered that the solvents may be released out of CNTs by heating organic molecules together with organic solvents, thus resulting in the encapsulation of the organic molecules in the free space appearing inside the CNTs.
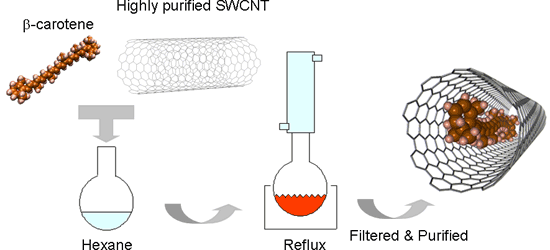
Carotenoid pigments, which are π-conjugated molecules, have large third-order optical nonlinearity and ultra-fast responsiveness. Thus, they are industrially valuable, but degradable by oxidization and isomerization. We heated a saturated hexane solution of β-carotene (β-carotene of 70 mg to hexane of 40 mg) containing open-ended SWCNTs (1 mg) in the presence of oxygen at approximately 70°C for 10 hours. Then, we repeatedly carried out ultrasonic cleansing and filtration of the solution using a tetrahydrofuran solution to remove β-carotene adhering on the outer side of the tubes (Fig. 2).
In this study, as the SWCNT, we used the "HiPco tube" available commercially from Carbon Nanotechnologies Inc. as well as tubes we produced by a laser evaporation method.
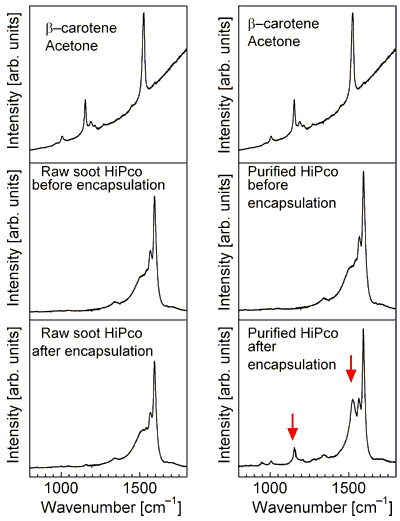
With unpurified close-ended HiPco tubes, the Raman spectra of encapsulation-processed samples showed almost no signals characteristic of β-carotene, but with purified HiPco tubes whose ends are made open by hydrochloride (HCl) etching or heating in air, the spectra of encapsulation-processed samples clearly showed signals characteristic of β-carotene (Fig. 3).
From the absorption spectra of highly purified, open-ended SWCNTs which are produced using a laser evaporation method, we have found that β-carotene can be encapsulated in the SWCNTs of 30 %.
Furthermore, with dimethylformamide (DMF) solutions of β-carotene and DMF solutions in which β-carotene-including SWCNTs are dispersed, we have observed change in absorption bands characteristic of β-carotene due to the radiation of an ultra-violet light (365 nm, 90 W) for 30 minutes. For the solution of β-carotene alone, the characteristic absorption bands disappear, while for the solution of β-carotene included in the SWCNTs, the bands showed almost no change. This may suggest that the light-degradation of β-carotene can be suppressed by the prevention from the attack of oxygen and radical species due to the SWNCT wall. In addition, we have found that β-carotene encapsulated in SWNCTs has greater heat-resistance than that dispersed in a polymer (polymethylmethacrylate, PMMA).
In this study, we have succeeded in the encapsulation of β-carotene in SWCNTs at a temperature of 70°C, fairly close to room temperature, and confirmed that the light-degradation of β-carotene can be suppressed by the encapsulation.
 |
|
Figure 2 Encapsulation procedure of β-carotene in SWCNT |
 |
|
Figure 3 (Left) Raman spectra for HiPco tubes and/or β-carotene solutions. A Raman spectrum of an encapsulation-processed β-carotene solution with unpurified close-ended HiPco tubes show no signals characteristic of β-carotene, while a Raman spectrum of an encapsulation-processed β-carotene solution with purified open-ended HiPco tubes clearly show signals (marked by red arrows) characteristic of β-carotene. |
 |
|
Photo 1. (Left) A solution in which SWCNTs are dispersed. (Right) A solution in which SWCNTs including β-carotene are dispersed shows diffuse red color due to β-carotene. |