- Orientation Control and Fixation of Organic Nanocrystals by a Magnetic Field -
The Photonics Research Institute (PRI) of the National Institute of Advanced Industrial Science and Technology (AIST), an independent administrative institution, has succeeded in preparing anisotropic bulk materials with orientation of organic nanocrystals controlled and solidified in a magnetic field, in collaboration with Prof. Hachiro Nakanishi group, the Institute of Multidisciplinary Research for Advanced Materials, Tohoku University (Tohoku U., hereinafter), a national university corporation. (Figs. 1 and 2)
In the field of organic optoelectronics, it has been urgently demanded to control the orientation and alignment of organic nanocrystals for the purpose of acquiring innovative functions. In contrast to inorganic materials, however, no technology has been available to orient organic nanocrystals effectively.
In the present research, a nanocrystals dispersion of organic compounds was prepared successfully with an acrylate monomer as a dispersion medium, through the "reprecipitation" method which is a simple, adaptable and excellent preparatory process for organic nanocrystals.
The prepared acrylic resin has thermally stable anisotropy despite its rubber-like solid, and can be prepared in a bulk of centimeter size, which a feat has never been achieved until now. Moreover, the resin has a high degree of freedom, allowing us to produce resin of diversified shape.
The novel method of orientation control and fixation for organic nanocrystals is applicable to other organic compounds, opening many applications not only for organic optoelectronics, but also for other advanced technologies.
The present R&D works have been supported by the Japan Science and Technology Agency (JST), another independent administrative institution, under the CREST Basic Research Program "Creation and Functions of New Molecules and Molecular Assemblies" (Fiscal Years 2000-2005).
Details of the R&D work has been already published in the January 31 issue of the science journal, Advanced Materials, 2005 WILEY-VCH Verlag GmbH & Co. KgaA, Weinheim.
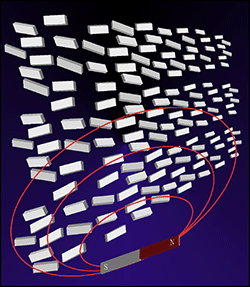
Fig. 1. Orientation of organic nano-crystals in a magnetic field
|
|

Fig. 2. Anisotropic bulk materials of various sizes
|
Organic materials are expected to be applicable in diversified areas owing to their wide array of structures. As an innovative devices is close to appear, taking advantage of specific merits of organic materials, such as printable thin film transistor (TFT) and flexible electroluminescence (EL) display, the interest in organic optoelectronics becomes recently increasing.
In the field, effective control of direction and alignment of organic molecules (orientation control) has attracted a great deal of public attention. It is anticipated that new functions can be created through the orientation control of organic molecules in those fields such as organic semiconductors, organic light emitting devices, organic non-linear optical materials, photonic crystals, et al.
There is no technology, however, to effectively control the orientation of organic molecules in a bulk rod of centimeter size for those purposes. It is not deniable that the technology for preparing organic anisotropic bulk materials is immature in contrast to inorganic materials. Therefore, for achieving a significant leap forward in the field of organic optoelectronics, technology for preparing organic bulk materials with effective orientation control has been required as well as for inorganic materials.
At the Institute of Multidisciplinary Research for Advanced Materials, Tohoku U., it has been successfully carried out to prepare various kinds of organic nanocrystals through an excellent process of the "reprecipitation" method and to observe the orientation of anisotropic nanocrystals along the electrical field applied to nanocrystals dispersion consisting of organic molecules DAST (trans-4-[4-(dimethylamino)]stilbezolium-p-toluenesulfonate) having large dipole moment, which was prepared by the reprecipitation method.
Meanwhile, the PRI-AIST has observed the magnetic field-induced orientation of DAST nanocrystals dispersion caused by the diamagnetic interactions. The magnetic field also has the potential to orient molecular aggregates such as nanocrystals with dozens of nanometer size (1 nm = 10-9 m) although the magnetic orientation of isolated molecules is impossible to observe because the thermal motion energy is generally greater than the orientation energy for a molecule. As the magnetic field-induced orientation is also useful for crystals without large dipole moment, it plays a complementary role for electrical field-induced orientation. The magnetic field-induced application is readily useful for bulk-sized dispersion systems, in spite of size, shape of sample, and all kind of dispersion mediums including water.
An organic nanocrystal is an aggregate of organic molecules, which are orderly arranged in alignment. On the other hand, organic nanocrystals dispersion contains a great number of organic nanocrystals in random orientation. In the bulk, it seems that organic molecules are disarrayed. If each organic nanocrystals in the dispersion could be arrayed, it would be possible to prepare a material with molecules oriented in a bulk of centimeter or larger size. It has been already demonstrated that organic nanocrystals can be oriented in an electrical or a magnetic field. The orientation of organic nanocrystals are disarrayed when we stop to apply the field. It is necessary, therefore, to develop technologies for fixing crystalline orientation.
The DAST nanocrystals dispersion was prepared by injecting ethanol solution of DAST containing a cationic surfactant, n-dodecyl-trimethyl ammonium chloride, into a dispersion medium, lauryl acrylate monomer (Fig. 3).
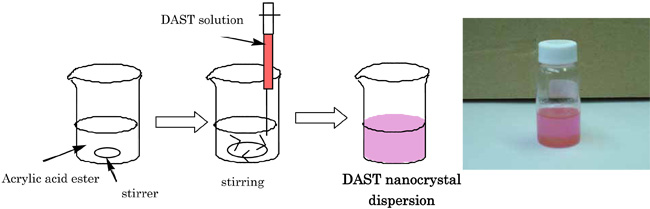 |
Fig. 3. Preparation of DAST nano-crystalline dispersion through the "reprecipitation" method. |
The anisotropic orientation and fixation of the DAST nanocrystals dispersion system has been carried out in a superconducting magnet, which can generate magnetic flux density as high as 17 tesla (T), by ultraviolet light in nitrogen atmosphere with benzoin isopropyl ether as a photo-initiator (Fig. 4).
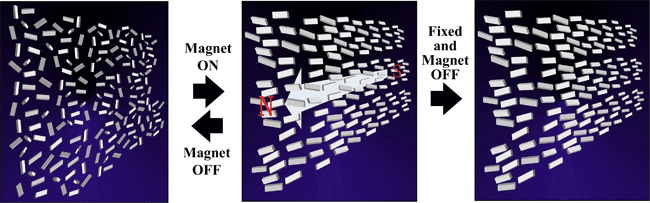 |
Fig. 4. Process of fixing organic nanocrystals dispersion in anisotropic orientation in a magnetic field. |
The fixation of DAST nanocrystals dispersion results in red-colored, transparent rubber-like solid. The fixed orientation is thermally very stable, without causing any change for six months or longer at room temperature, and 24 hours or longer even at 100 ºC. Fig. 5 shows polarized absorption spectrum of DAST nanocrystals dispersion photocured in 15 T, in contrast to spectrum (C) without applying magnetic field. The maximum contrast in the absorption intensity at 555 nm between samples oriented with polarization angles of 0 degree (parallel) and 90 degree (perpendicular) with respect to the direction of the applied magnetic field was about 0.4, which can be readily discriminated visually if a polarizer is used (Fig. 6).
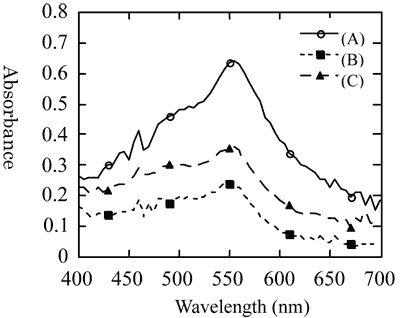 |
Fig. 5. Polarized absorption spectra of DAST nanocrystals dispersion with anisotropic orientation solidified in 15 T. |
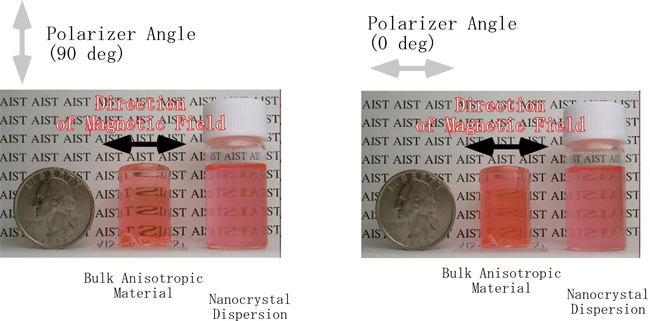 |
Fig. 6. Pictures of DAST nanocrystals dispersion with anisotropic orientation photocured in 15 T.
Left: The polarizer was rotated to make the middle sample as transparent as possible.
Right: Rotated 90 degree from the position of the left picture.
|