In collaboration with the New Glass Forum (NGF) and Himeji Institute of Technology, the Special Division for Green Life Technology—part of the National Institute of Advanced Industrial Science and Technology (AIST)—has developed a novel inorganic–organic hybrid conductive membrane by introducing conductive organic molecules into the nanopores of a porous glass previously developed by the AIST. The membrane is highly proton conductive in the presence of high-temperature water vapor. The conductive membrane structure consists of organic molecules introduced into an inorganic skeleton. It is resistant to heat and organic solvents, and is expected to exhibit high proton conductivity as the conduction path exists as nanopores. The research may significantly accelerate the development of novel fuel cells and sensors.
Conventional Nafion is difficult to use at high temperatures (> 100 ºC). Therefore, it cannot be used with waste heat or other applications and is associated with a number of problems including poor energy efficiency. Researchers have been pursuing the development of a solid electrolyte membrane that can be used at > 100 ºC.
The AIST team investigated the use of porous glass containing nanopores in the development of a material resistant to heat and organic solvents. They successfully developed a novel inorganic–organic hybrid conductive membrane by introducing conductive organic molecules into the nanopores.
In the future, the research team plans to develop a more highly conductive membrane by controlling nanopore orientation.
This work was supported by the "Nano technology glass project" from New Energy and Industrial Technology Development Organization (NEDO) of Japan.
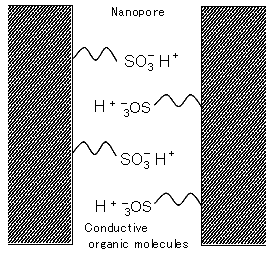
Figure 1. Schematic diagram of the inorganic–organic hybrid conductive membrane
|
|
Temperature (ºC) |
Conductivity (S/cm) |
|
105 |
1.6×10-2 |
|
110 |
2.3×10-2 |
|
115 |
2.8×10-2 |
|
120 |
4.2×10-2 |
Relative humidity: 100%
Table 1. Conductivity of the inorganic–organic hybrid conductive membrane
|