– Realizing highly difficult C-H bond cleavage and a selective reaction with a semiconductor photoelectrode that uses sunlight –
Researchers: SAYAMA Kazuhiro, Prime Senior Researcher, Research Center for Photovoltaics, and MISEKI Yugo, Senior Researcher, and TATENO Hiroyuki, AIST Postdoctoral Researcher, Advanced Functional Materials Team of the center
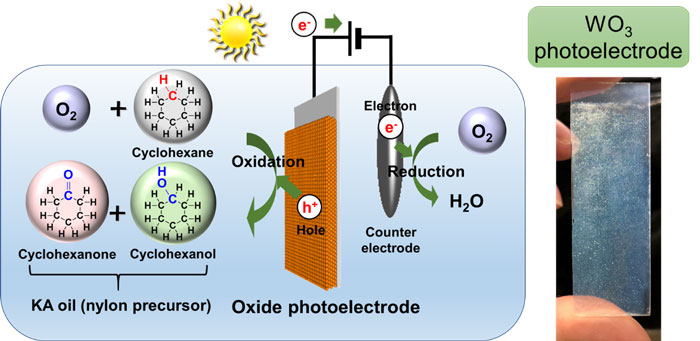
The researchers have developed a technology for synthesizing KA oil (a mixture of cyclohexanone and cyclohexanol), a raw material of nylon, etc., at room temperature and atmospheric pressure by direct oxidization of cyclohexane using sunlight with an oxide semiconductor photoelectrode.
 |
|
Schematic diagram of KA oil synthesis using sunlight and an oxide photoelectrode (left) and photograph of a photoelectrode (right) |
Synthesis of KA oil by oxidation of cyclohexane is an important process in nylon production, and it is produced using a large amount of energy at high temperature and high pressure. It is an oxidation reaction that is difficult to improve selectivity, so there is demand for an innovative process. Expectations are great for chemical production processes that directly use sunlight with a photocatalytic electrode. AIST has developed an efficient method for synthesizing inorganic oxidants using an oxide semiconductor photoelectrode.
The researchers prepared a semiconductor photoelectrode with a tungsten oxide thin film by spin-coating and baking a precursor solution on conductive glass. They placed the semiconductor photoelectrode and a counter electrode in a reaction vessel filled with a solution containing cyclohexane, nitric acid, and dissolved oxygen. When they applied simulated sunlight with a slight external voltage of 2 volt or less, KA oil was synthesized with a selectivity of 99% or above, at room temperature and atmospheric pressure. The external voltage had the effect of greatly increasing production speed, and the reaction does not proceed until light is irradiated on the electrode.
With the aim of photoelectrochemical production of chemicals that greatly reduce the external voltage required for reactions using sunlight, the researchers will develop various organic and inorganic reactions with high efficiency.