- Toward practical use as a fluorescent reagent for bio-applications utilizing its high brightness and durability -
Norio Murase (Senior Research Scientist) and Masanori Ando (Senior Research Scientist), Advanced Health Research Group (Leader: Yasushi Shigeri), the Health Research Institute (Director: Yasukazu Yoshida) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have developed photoluminescent glass capsules (diameter: 20 to 100 nm) containing multiple CdSe/ZnS core/shell quantum dots (QDs) (semiconductor nanocrystals) that retain their photoluminescence (PL) properties. With high emission brightness and the durability of glass, the capsule can be used as a fluorescent reagent in a wide variety of bio-applications, from basic research to clinical applications. Its brightness and durability could make it useful as a phosphor for electronics.
Organic polymer-coated QDs are commercially available as fluorescent reagents for research purposes that are used to stain cells and observe their form, distribution, and movement. However, these dots are individually polymer-coated, making it impossible to increase the brightness. Because the polymers lack high chemical durability, the polymer-coated QDs are susceptible to the elution of constituents or degradation of the QD in bio-applications. They therefore have limited applications. Because it is advantageous to encapsulate the QDs in glass which is more durable than polymers, studies on glass-encapsulated QDs have been conducted around the world. However, nobody has yet succeeded in developing them.
We have developed a three-step synthesis based on a sol-gel method and have succeeded in encapsulating CdSe/ZnS core/shell QDs in glass capsules at high concentration. The CdSe/ZnS core/shell QDs have a higher PL efficiency and a narrower spectral width than other photoluminescent QDs, and the QDs retain their properties in the capsules. As a result, we have obtained a bright, highly light-resistant fluorescent reagent that is less susceptible to leaching of cadmium.
 |
|
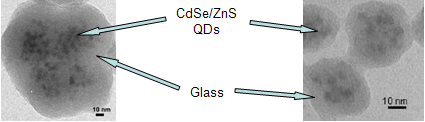
Photo: TEM image of bright photoluminescent glass capsules containing many dispersed QDs
The diameter of capsules can be controlled by the reaction conditions. (Left: 95 nm particle diameter; right: 40 nm particle diameter)
|
In bio-applications, a fluorescent reagent is bound to a cell or a biological substance to investigate its form, amount, distribution, and movement inside and outside a living body. Conventionally, organic fluorescent reagents are used. A QD with a diameter of 2 to 10 nm has high PL efficiency and very high resistance to light, and therefore is expected to be used as a high-performance fluorescent reagent. The QD has absorption and emission wavelength ranges far apart from each other, making it easy to detect light emission. The emission wavelength can be adjusted by changing the particle size.
However, QDs easily agglomerate and precipitate owing to their large specific surface area. To prevent this, technology has been developed to stabilize QDs by coating them with a transparent material and thus improving their dispersion in solvents. Polymer-coated QDs are commercially available, but it is difficult to increase further their emission brightness. In bio-applications, QDs are diluted and dispersed in a high-salt-concentration solution and irradiated with strong ultraviolet (UV) light as the excitation light. Under these severe conditions, problems occur, such as decreased emission brightness due to leaching of the constituents or the degradation caused by UV light, and cell death through the release of toxic constituents such as cadmium. Because of this, there is demand for bright, highly durable QD-dispersed phosphors with a particle diameter of less than 100 nm for easy endocytosis.
Over the past 10 years, extensive studies have been conducted to achieve both bright emission and high durability (chemical durability and light resistance) by dispersing the QDs at a high concentration in glass, which is more durable than polymers. One example is silica microspheres (diameter: several hundred nm) with a QD-dispersed glass layer near the surface. Another example is glass capsules containing a single QD. However, both types have a low QD concentration, making it difficult to increase emission brightness. The latter type requires the addition of a thick shell to the QD to maintain its PL efficiency; this is a laborious task. For these reasons, these types of capsule are not sufficient to use as fluorescent reagents.
The use of a QD as a fluorescent reagent for bio-applications involves the problems of low emission brightness due to a very low concentration and early degradation due to high excitation-light intensity. It has been found that, among QDs, a CdSe/ZnS core/shell QD is advantageous in increasing emission brightness because it absorbs excitation light well and is relatively stable in buffer solutions. AIST has worked to develop a bright, highly durable (i.e. with chemical durability and light resistance) QD-dispersed photoluminescent glass capsule with a particle diameter of less than 100 nm for easy endocytosis. The goal was to develop a technology to produce a bright, highly durable, photoluminescent glass capsule with CdSe/ZnS QDs dispersed at a high concentration. The technology was based on a sol-gel method for producing glass by the hydrolysis of silicon alkoxide.
This study was supported in part by the Creation and Support Program for Start-ups from Universities (2007-2010), Project to develop “Innovative Seeds”, sponsored by the Japan Science and Technology Agency.
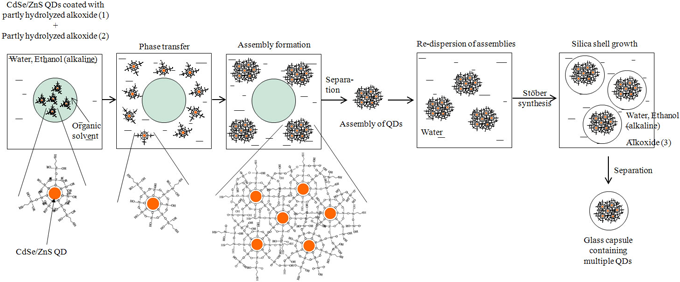
Requirements for a fluorescent reagent include high emission brightness, resistance to degradation and leaching of the constituents when dispersed in a buffer solution for bio-applications, resistance to light degradation when irradiated with intense UV light (excitation light), and small size (less than 100 nm) for easy endocytosis. The capsule must contain more than ten QDs to achieve high brightness and therefore should be larger than about 20 nm. We found that the Stöber synthesis (sol-gel method) is suitable for obtaining the desired 20- to 100-nm glass capsule, and we have improved this method. Specifically, QDs are encapsulated in a durable glass capsule in such a way that their high PL efficiency is retained. First, the QDs are properly coated to increase durability. Then, assemblies of the coated QDs are formed and a photoluminescent glass capsule is produced by depositing silica on the surface of the assembly. The details of the three-step synthesis (Fig. 1) are described below:
[Step 1] Add silicon alkoxide (1) to an organic solvent containing CdSe/ZnS QDs. Partly hydrolyze silicon alkoxide (1) and coat the QDs with the hydrolysate (organic solution A). Generally, the PL efficiency of a QD is significantly affected by the surface condition of the QD. This coating prevents a decrease in the PL efficiency of the CdSe/ZnS QD.
[Step 2] Prepare an aqueous solution containing partly hydrolyzed silicon alkoxide (2). Mix this with organic solution A prepared in step 1 to form a layer of alkoxide (2) on the surface of the QDs coated with alkoxide (1). The silicon alkoxides on the surface of the QDs are further hydrolyzed upon contact with water. The QDs become hydrophilic and move to the aqueous phase, forming assemblies. Select appropriate species and concentrations of silicon alkoxides to make the hydrolysis of alkoxide (2) slower than that of alkoxide (1) and thereby prevent the formation of large aggregates of the QDs. As a result, a decrease in PL efficiency due to imperfect chemical bonds between the QDs can be prevented.
[Step 3] Deposit a silica (glass) layer on the surface of the QD assembly to produce a QD-dispersed photoluminescent glass capsule. This can be done by hydrolyzing silicon alkoxide (3) in an alkaline solvent by the Stöber synthesis and then depositing the alkoxide around the QD assembly. In this step, a dense silica layer is deposited to coat the QD assembly, making the produced photoluminescent glass capsule highly durable.
 |
|
Figure 1: Production by the three-step process of a bright, highly durable glass capsule with an assembly of CdSe/ZnS QDs as the core |
A 20- to 100-nm photoluminescent glass capsule is obtained by depositing highly networked silica around densely assembled CdSe/ZnS core/shell QDs using the three-step synthesis that we developed. This glass capsule contains more than ten CdSe/ZnS QDs. Capsules of different diameters (e.g. 40 and 95 nm, shown in Fig. 2) can be produced by changing the concentration of silicon alkoxide (1) and (2) used in the steps 1 and 2. The PL efficiency of the encapsulated QD is similar (20% to 35%) to that of the non-encapsulated QD. Transmission electron microscopy (TEM) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) showed that, for example, a 47-nm capsule contains about 25 CdSe/ZnS QDs. The emission brightness of a photoluminescent glass capsule is proportional to the product of the PL efficiency and the concentration of QDs. The produced photoluminescent glass capsule contains a high concentration of QDs with high PL efficiency. As presumed, the photoluminescent glass capsule is very bright.
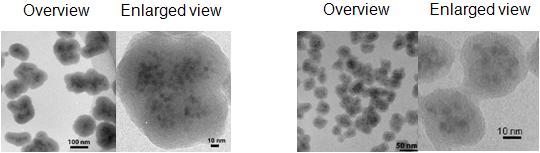 |
Figure 2: TEM images of the photoluminescent glass capsules with many dispersed CdSe/ZnS QDs
Left: A large capsule (95 nm in diameter)
Right: A small capsule (40 nm in diameter) |
The photoluminescent glass capsule is resistant to the elution of the constituents and degradation when diluted to 10 nM (nmol/L) (the concentration of the QDs) in a buffer solution for bio-applications. The amount of cadmium released from the glass capsules in a high-salt-concentration HEPES buffer solution was less than one-tenth of that from commercially available polymer-coated CdSe/ZnS QDs; thus, the capsule was highly durable. Resistance to UV light irradiation was 100 times that of polymer-coated CdSe/ZnS QDs.
It is possible to introduce functional groups including a carboxyl group into the photoluminescent glass capsule by the chemical modification of its surface. This technique allows an antibody to be attached to the surface of the photoluminescent glass capsule, making it a fluorescent probe for observing specific molecules in a living body.
The light emitted from individual QDs is known to blink. When QDs are used as fluorescent reagents, the blinking of bright spots might be misinterpreted as indicating movement of the substance labeled with the reagent. Therefore, less blinking is desirable. Trajectories (time dependence) of the PL intensity emitted from a single CdSe/ZnS QD and a single glass capsule (containing an average of 15 CdSe/ZnS QDs) were compared using fluorescence microscopy. Blinking is clearly observed with the single QD, but not with the glass capsule. Because many QDs are contained in the capsule, the light emitted from the QDs is averaged out, removing the blinking and giving stable emission intensity (Fig. 3). As expected from the number of dispersed QDs, the emission intensity of a single glass capsule is about 15 times that of a single QD. Thus, the photoluminescent glass capsule has been demonstrated to be advantageous as a fluorescent reagent in terms of emission stability.
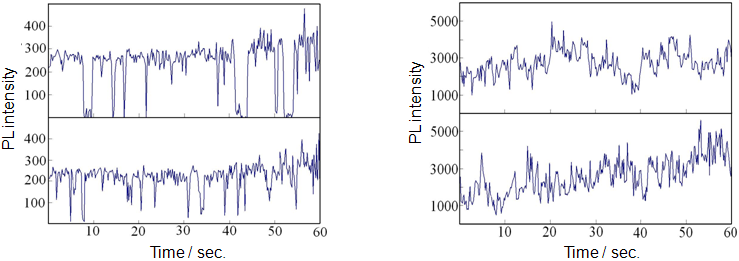 |
Figure 3: Left: PL-intensity trajectories of a single CdSe/ZnS QD
Right: PL-intensity trajectories of a single glass capsule containing an average of 15 CdSe/ZnS QDs |
We will study the feasibility of mass production of the glass capsules for a wide variety of bio-applications, from fluorescent reagents for cells and biological substances used in basic research to clinical applications including the quick diagnosis of infectious diseases, and we will propose collaboration with the manufacturers of related products with a view to setting up a venture business. We will also develop uses of the capsule as a phosphor in electronics.