– Demonstrated "continuous" hydrogen production from formic acid for over 2,000 hours –
Researchers) KAWANAMI Hajime, Chief Senior Researcher, Functional Group Transformation Team, Interdisciplinary Research Center for Catalytic Chemistry
- Highly efficient "continuous" conversion of formic acid to hydrogen using a flow reaction system
- Over 5 hour stable power generation using a fuel cell with generated hydrogen from formic acid
- The generated hydrogen will be applied to various utilizations other than power generation.
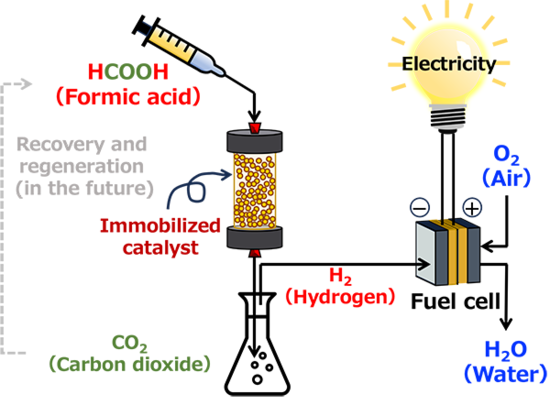
"Continuous" hydrogen production process from formic acid by flow system
In recent years, with the increasing focus on the realization of a hydrogen society to address energy and global warming issues, the technological development of hydrogen carriers for efficient storage, transportation, and production of hydrogen has been progressing. AIST has been conducting a series of studies on using formic acid as a hydrogen carrier. Formic acid, which is used as an additive in dairy farming among other applications, is a relatively easy-to-handle chemical. While formic acid is industrially produced from methanol and other sources overseas, its synthesis methods from carbon dioxide, biomass, and methane are being developed. As a result, formic acid stands out as a hydrogen source that can contribute to reducing carbon dioxide emissions. However, there were many challenges in implementing formic acid as a hydrogen carrier in societal use.
A researcher at AIST, in collaboration with the University of Tsukuba, has developed a power generation system using hydrogen derived from formic acid with a flow-type reaction system.
Formic acid is considered one of the promising hydrogen sources to address future energy challenges. It is derived from biomass, carbon dioxide, etc., and is primarily used as an animal feed additive in grass silage. There are many challenges to implementing the technology to produce hydrogen from formic acid, and there have been only a few practical demonstrations of this technology in Japan. Thus, in this research, the catalyst for hydrogen production from formic acid was revisited, and a heterogeneous catalyst consisting cross-linked polyethyleneimine with iridium complexes and uncoordinated bipyridine ligands was newly designed and synthesized for the flow-type "continuous" hydrogen production process through formic acid dehydrogenation. We conducted a power generation test using a fuel cell and successfully demonstrated stable electric power generation for over 5 hours.
These achievements are expected to pave the way for the implementation of energy technology that utilizes formic acid as a liquid organic hydrogen carrier. Simultaneously, the generated hydrogen from formic acid is expected to be used for various applications beyond power generation.