– Oriented to high performance ammonia synthesis integrated with electrolytic hydrogen production using renewable energy –
Researchers) NISHI Masayasu, Senior Researcher, CHEN Shih-Yuan, Senior Researcher, TATENO Hiroyuki, Researcher, MOCHIZUKI Takehisa, Group Leader, Energy Catalyst Technology Group, Energy Process Research Institute, TAKAGI Hideyuki, Team Leader, Hydrogen Production and Storage Team, Global Zero Emission Research Center, NANBA Tetsuya, Deputy Director, Research Center, Fukushima Renewable Energy Institute
- Development of a highly active catalyst for ammonia synthesis that can operate even under fluctuating conditions with quick startup and shutdown operation.
- Ammonia yield is more than 1.5 times higher than that of conventional catalysts
- This new catalyst would contribute to practical application of ammonia synthesis process using renewable energy and its products, such as electrolytic hydrogen production under intermittent supply conditions.
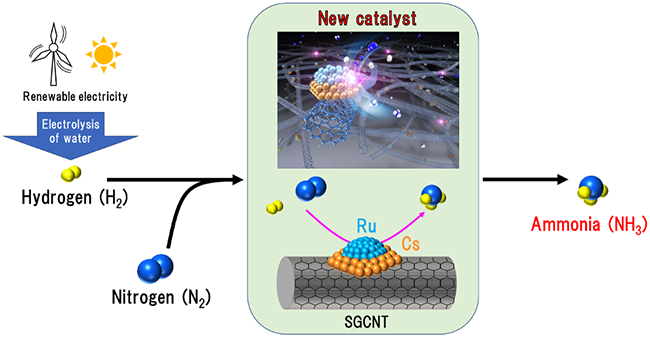
Integration of hydrolytic hydrogen production with ammonia synthesis using renewable energy
Ammonia does not contain carbon atoms in its molecule and does not emit CO2 even when burned, so technology that uses ammonia as a fuel to replace fossil resources has attracted attention. The Haber-Bosch process is an industrial process for artificial synthesis of ammonia via the reaction of fossil-based hydrogen and nitrogen catalyzed under high temperature and pressure conditions (400-600 °C and 100-300 atm). This process emits large amounts of CO2 especially during the production of fossil-based hydrogen. Therefore, it is imperative to develop new process, which can perform the ammonia synthesis using nitrogen derived from the air and green hydrogen derived from electrolysis of water using renewable electricity such as solar and wind power.
Generally, the amount of renewable electricity fluctuates depending on the weather and geographic conditions. Thus, the electrolytic hydrogen production via electrolysis of water using renewable electricity also fluctuates. It is therefore that the ammonia synthesis plants would stop and resume the operation conditions because there is the time when the electrolytic hydrogen production cannot be supplied. Thus, the catalyst for ammonia synthesis using electrolytic hydrogen should have high performance even under fluctuated operation conditions. For energy saving, it is also crucial to develop the catalyst that can efficiently catalyze ammonia synthesis under lower temperature and pressure as compared with the industrial Haber-Bosch process.
Researchers in AIST developed a new catalyst that was suitable for integrated process of electrolytic hydrogen production and ammonia synthesis using renewable energy.
This catalyst consists of ruthenium (Ru) and cesium (Cs) supported on the single-walled carbon nanotubes produced by the super-growth method (termed SGCNT). This Cs-Ru/SGCNT catalyst can stably synthesize ammonia even under fluctuated operations conditions, particularly those with fast stop and resume to meet the supply of electrolytic hydrogen production. In addition, the ammonia yield was 1.5 times higher than the reported catalysts in the literature even though the reaction temperature and pressure were lower than the conventional conditions. The electrolytic hydrogen production is highly related to the supply of renewable energy affected by the weather and geographic conditions. However, this catalyst can integrate electrolytic hydrogen production and mild ammonia synthesis using renewable electricity and its products, which might largely reduce carbon footprint and thus provide an alternative way for green ammonia synthesis.