- Contribution to the elucidation of pathogenic mechanisms and the screening for candidate molecules of therapeutic drugs –
Tomoyo Ochiishi (Senior Researcher) and others, the Molecular Neurobiology Group, the Biomedical Research Institute (Director: Yoshihiro Ohmiya), the National Institute of Advanced Industrial Science and Technology (AIST; President: Ryoji Chubachi), Akira Kitamura (Assistant Professor), the Faculty of Advanced Life Science, Hokkaido University (President: Keizo Yamaguchi), Hideki Shimura (Associate Professor), Department of Neurology, Juntendo University School of Medicine (CEO: Hideoki Ogawa), and others have developed a technology to visualize the molecular dynamics of amyloid β (Aβ) protein, one of the causative factors of Alzheimer’s disease, in live neurons or in vivo.
Aβ easily polymerizes and then forms a large aggregate. The GFP fluorescence from a fusion protein of Aβ and a fluorescent protein GFP (Aβ-GFP) is thought to be inhibited upon Aβ aggregation. Therefore, even when Aβ-GFP is expressed in vivo, the fluorescence is not detected once Aβ aggregates and it is difficult to visualize the localization or dynamics of Aβ. In the present study, however, the researchers modified the amino acid sequence (the linker) between Aβ and GFP and developed a fusion protein that maintains GFP fluorescence regardless of the Aβ aggregation status. They also revealed that this fusion protein forms an Aβ oligomer, which is toxic and is involved in the pathogenesis of Alzheimer’s disease.
This technology is anticipated to contribute to the screening for candidate molecules of therapeutic drugs for Alzheimer’s disease using cultured cells or live animals and to the elucidation of the pathogenic mechanisms of this disease. Details of the results will be published in Scientific Reports (an open-access online journal) on March 16, 2016.
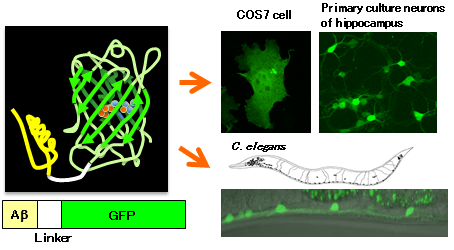 |
|
Schematic diagram of the developed Aβ-GFP fusion protein (left) and its expression in cultured cells and the nematode Caenorhabditis elegans (right) |
Related to the current status of medical expenses and the nursing system, an increase in the number of patients with dementia in an aging society is a major social problem worldwide. Alzheimer’s disease, which is characterized by memory loss, impaired capacity for judgment, disorientation, etc., accounts for more than half the cases of dementia. However, details of its pathogenic mechanisms have not been revealed and effective therapies or drugs have not been developed.
AIST has promoted research to elucidate molecular functions and structures related to biological mechanisms or diseases. Based on findings in such research, AIST has developed fundamental technologies for drug discovery and healthcare. Alzheimer’s disease is a type of dementia, which is pathologically characterized by perineuronal deposition of senile plaques that mainly consist of Aβ, neurofibrillary tangles caused by massive intraneuronal accumulation of hyperphosphorylated tau proteins, and brain atrophy. These pathological changes are potential causes of this disease; its pathogenic mechanisms, however, have not been revealed. Recently, a new hypothesis has been widely accepted: a few Aβ molecules aggregate to form an Aβ oligomer, which is highly cytotoxic, and the intracellular accumulation of these oligomers is strongly associated with the pathogenesis of Alzheimer’s disease. Conventional methods were only capable of analyzing collected samples of cells and brain tissues. New methods were therefore needed to directly visualize the status of Aβ oligomerization in live cells for in-depth analysis of the causal relationship between such a status and cytotoxicity, and to directly analyze the effects of therapeutic drug candidates against Aβ aggregation. Thus, collaborative research was conducted between AIST (which has been working with visualizing cells, analyzing neuronal functions, and generating and analyzing transgenic animals) and the Faculty of Advanced Life Science of Hokkaido University (which has achieved outstanding outcomes in analyzing molecular interactions in live cells with fluorescence correlation spectroscopy).
Previous studies reported various types of Aβ-GFP that contain a linker between Aβ and GFP of 12 or fewer amino acids. When Aβ aggregated, however, these fusion proteins lost GFP fluorescence, rendering detection of cytotoxic oligomers problematic. In this study, the researchers generated a linker of 14 amino acids and developed the Aβ-GFP fusion protein that maintained GFP fluorescence even after Aβ formed an aggregate. Analyses of this Aβ-GFP by nuclear magnetic resonance, electron microscopy, immunohistochemistry, and fluorescence correlation spectroscopy revealed that the fusion of Aβ with GFP prevented Aβ aggregation after a certain stage and that the Aβ-GFP remained as oligomers, ranging mainly from dimers to tetramers, in vivo and in vitro (Fig. 1). These characteristics can be used for analysis of the Aβ dynamics in live cells or the accumulation status of Aβ in primary cultured neurons. The relationship between the Aβ aggregation status and cytotoxicity can also be analyzed, because an Aβ oligomer is more cytotoxic than polymerized Aβ in a fibrillar form.
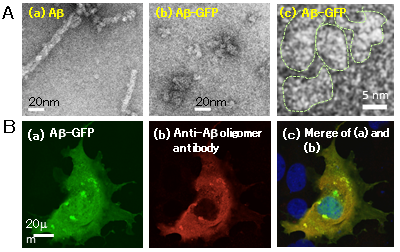 |
Figure 1: Expression of Aβ-GFP in vivo and in vitro
(A) Electron microscopic images of Aβ (a) and Aβ-GFP (b and c). Whereas polymerized Aβ forms fibrils (a), Aβ-GFP only forms oligomers (in panel (c), an area surrounded by a dotted line indicates each Aβ aggregate).
(B) COS7 cell expressing Aβ-GFP (a) stained with an antibody that recognizes only Aβ oligomers (b). Panel (c) shows that the staining patterns in panels (a) and (b) are almost identical, suggesting that Aβ-GFP exists as oligomers. |
When the linker between Aβ and GFP is short, Aβ aggregation eliminates GFP fluorescence. Based on the phenomena observed earlier in this research, the researchers thought out a system to detect the Aβ aggregation status and demonstrated that GFP fluorescence intensity could be used to screen for candidate molecules of therapeutic drugs (Fig. 2). When GFP was expressed in a certain type of neurons in C. elegans, bright fluorescence was observed in these neurons (Fig. 2B (a)). On the other hand, when Aβ-GFP with a short linker of two amino acids was expressed in C. elegans, GFP fluorescence was not observed after Aβ aggregation (Fig. 2B (b)). When these Aβ-GFP transgenic nematodes were cultured with curcumin, which suppresses Aβ aggregation, GFP fluorescence was detected (Fig. 2B (c)). Thus, a change in fluorescence intensity of Aβ-GFP can be used to screen for drug candidates that suppress Aβ aggregation.
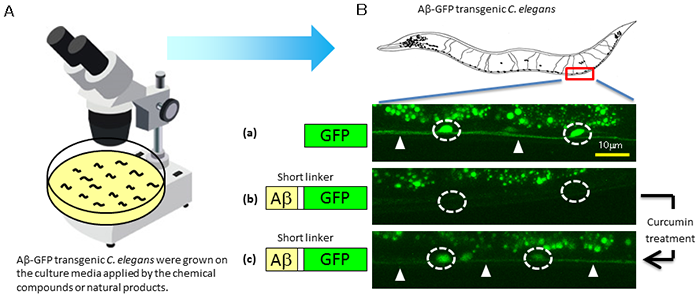 |
Figure 2: Example of screening for drug candidates in vivo
Microscopic observation of C. elegans on culture medium (A) demonstrates that suppression of Aβ aggregation by curcumin enhances the fluorescence intensity in neurons (B). In panel (B), white-lined circles indicate the cell bodies of neurons and arrowheads indicate the axons. |
Using the system to detect Aβ-GFP fluorescence intensity in cultured neurons, the researchers will begin development of an easy screening method for candidate molecules of therapeutic or preventive drugs for Alzheimer’s disease. They will also investigate the pathogenic mechanisms of this disease and conduct prevention research by the in-depth analysis of the effects of Aβ oligomers on subtle changes in neurons at a very early stage of Alzheimer’s disease using transgenic mice expressing the developed Aβ-GFP fusion protein.