Masahiro Asada (Senior Researcher), Toru Imamura (Leader), and others, Signaling Molecules Research Group, the Biomedical Research Institute (Director: Yoshihiro Ohmiya) of the National Institute of Advanced Industrial Science and Technology (AIST; President: Tamotsu Nomakuchi), have created novel cell growth factor FGFC that is considered effective in preventing and treating injury due to high-dose radiation, in collaboration with Makoto Akashi (Executive Director), the National Institute of Radiological Sciences (NIRS; President: Yoshiharu Yonekura) and Fumiaki Nakayama (Senior Researcher), Advanced Radiation Biology Research Program (Program Leader: Takashi Imai), the Research Center for Charged Particle Therapy, NIRS.
Until now, there were insufficient drugs effective in suppression of individual deaths due to radiation exposure. AIST has created the novel, highly stable cell growth factor FGFC and investigated its effect on serious impact to life caused by high-level radiation exposure by experiments on mice. Mice administered FGFC either before or after radiation exposure experienced prolonged survival, demonstrating that FGFC could be effective in preventing and treating fatal radiation injury. The researchers plan to perform a detailed safety evaluation of FGFC.
The details of these results will be presented at the 55th Annual Meeting of the Japan Radiation Research Society to be held at Tohoku University (Sendai, Miyagi) from September 6 to 8, 2012.
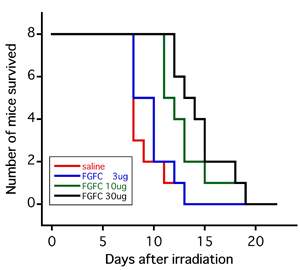 |
(Figure) : Survival curves of mice that received 8 Grays (Gy) of whole-body X-ray irradiation 24 hours following intraperitoneal administration of FGFC
The red line indicates the administration of physiological saline solution alone. The blue, green, and black lines indicate administration of 3, 10, and 30 µg of FGFC, respectively. |
Following the March 2011 accident at the Fukushima Daiichi Nuclear Power Plant of Tokyo Electric Power Co., Inc., society widely recognized the necessity of methods to prevent and treat radiation injury. However, few drugs of this kind have been known, for example potassium iodide, which inhibits accumulation of radioactive iodine in the thyroid gland, and G-CSF, which prevents white blood cell reduction and complications.
Palifermin (recombinant human keratinocyte growth factor FGF7; unapproved in Japan) is a drug approved in the U.S. for treating oral mucositis due to radiation therapy, and it is a member of the fibroblast growth factor (FGF) family. However, palifermin's range of effect (target cell specificity) is limited; for example, it only acts on epithelial cells. Also, this growth factor is unstable, requiring cumbersome repeat-dose treatment.
Considering this situation, the development of a more stable and widely applicable prevention and treatment drug for radiation injury has been anticipated.
AIST aims to uncover the function of signaling molecules that control the movement of cells and individual bodies and apply that knowledge toward novel treatment methods and identification of new target molecules of diagnosis, treatment, and drug development. In recent years, because the necessity of drugs for prevention and treatment of radiation injury is broadly recognized, research on cell growth factors for preventing and treating injury due to high-level radiation exposure has been promoted. In the past, FGF1, FGF7, and FGF10 were confirmed to minimize the reduction of the number of surviving jejunum crypt cells in mice following X-ray exposure and to have the effect of suppressing apoptosis marker expression in bone marrow. FGFC (optimized chimeric molecule of FGF1 and FGF2) was also created, and it has biological properties similar to those of FGF1 but is structurally more stable than FGF1.
As one part of the verification of the efficacy of FGFC as a protective drug against radiation injury, the effect of FGFC on the survival rate of individual mice exposed to high-dose radiation was investigated.
The experiments in the present study were conducted after investigation and approval by the animal experiment committee of AIST and conformed to animal experiment and test animal treatment guidelines.
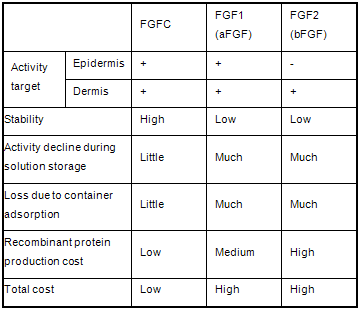
FGF activity is primarily conveyed to the inside of target cells through FGF receptors on the surface of those cells. Therefore, the physiological activity of FGF is decided by the expression regulation of FGF itself and the multiple corresponding FGF receptors and coreceptors. Palifermin (keratinocyte growth factor; FGF7) is specific to epithelial cells, and trafermin (basic fibroblast growth factor; FGF2), a drug that has been approved for the treatment of pressure ulcers, acts specifically on dermal cells. Meanwhile, acidic fibroblast cell growth factor FGF1 acts on a wide range of cells, but in order for its activity to be expressed, carbohydrate chains such as heparin are required. AIST has created various types of chimeric molecules in which parts of FGF1 and FGF2 were exchanged. One of those chimeric molecules that acts on wide range of cells and does not require heparin for growth is the cell growth factor FGFC (Fig. 1). FGFC possesses properties absent in conventional FGF, such as acid resistance, resistance to proteolytic enzymes, and adsorptivity (Table 1).
 |
|
Figure 1 : Schematic diagram of FGF1, FGF2, and FGF chimeric proteins (FGFC) |
|
Table 1 : Superiority of FGF chimeric protein FGFC |
 |
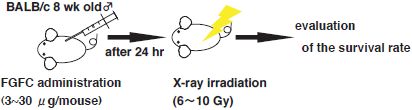
The effect of FGFC on the survival rate of individual bodies exposed to high-dose radiation was investigated as one part of the verification of the efficacy of the superior FGFC as a protective drug against radiation injury. BALB/c mice (approximately 8 weeks old; male; 8 mice per group) received intraperitoneal FGFC administration and underwent full-body X-ray irradiation 24 hours thereafter. The temporal change in the individual survival rate was then measured (Fig. 2).
 |
|
Figure 2 : Schematic diagram of experimental methods |
The results from studying the effect of X-ray radiation dose and FGFC dose on survival rate showed that when FGFC was administered in a range of 3 to 30 μg 24 hours prior to 8-Gy X-ray irradiation, post-irradiation survival days were increased as the administered FGFC dose increased (Fig. 3). Also, when mice were exposed to 6-Gy of X-ray irradiation, all of the individual mice administered 30 μg of FGFC survived, in contrast to the 38% of mice that died by 30 days following irradiation in the group administered only physiological saline solution. Meanwhile, no significant effect was confirmed when mice were exposed to 10-Gy radiation.
 |
Figure 3 : Survival curves of mice that received 8 Gy of whole-body X-ray irradiation 24 hours following intraperitoneal administration of FGFC
The red line indicates the administration of physiological saline solution alone. The blue, green, and black lines indicate administration of 3, 10, and 30 µg of FGFC, respectively. |
Next, the effect of FGFC as a treatment drug after radiation exposure was studied. FGFC was administered 2 or 24 hours after X-ray irradiation, and the effect on the survival rate was studied. In the group of mice exposed to 6-Gy radiation, survival rate was confirmed to have improved as a result of either administration of FGFC 2 hours or 24 hours after irradiation (Fig. 4). However, no significant effect was confirmed in the 8-Gy and 10-Gy irradiation groups.
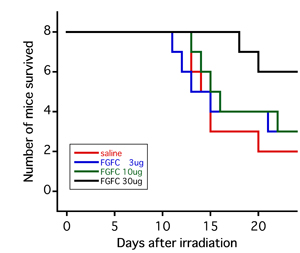 |
Figure 4 : Survival curves of mice that received intraperitoneal administration of FGFC 2 hours following 6 Gy of whole-body X-ray irradiation
The red line indicates the administration of physiological saline solution alone. The blue, green, and black lines indicate administration of 3, 10, and 30 µg of FGFC, respectively. |
As described above, FGFC administration was shown to be potentially effective in preventing and treating radiation injury when radiation dosage, FGFC dosage, and administration timing are within a certain range.
The researchers intend to analyze the mechanism of action of FGFC in more detail, investigate dose frequency and other concomitant procedures, and establish methods capable of maximally utilizing the effect of FGFC. They also plan to continue performing safety and other evaluations.