We have developed a new organic–dye sensitizer (MK-2
(Note 1)) as the optical absorption material for dye-sensitized solar cells (DSSCs) and investigated solar-cell performance and long-term stability of the DSSCs with MK-2. Unlike conventional ruthenium-complex sensitizers, this organic dye does not contain any rare metals such as ruthenium. Possibility of low cost production, high efficiency, and high long-term stability were shown by the solar cell.

Fig. 1 Organic-dye-sensitized solar cell prototype |
|
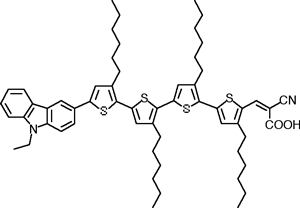
Fig. 2 Molecular structrure of MK-2
|
Kohjiro Hara and Nagatoshi Koumura, Research Scientists at AIST, have developed a high-performance dye-sensitized solar cell (DSSC) based on organic dye as part of the Grants-in-Aid Program for Scientific Research led by the New Energy and Industrial Development Organization (budget: about 5 billion yen).
This technology may provide a solution to both the high manufacturing cost of the currently predominant silicon solar cell and the unstable supply of high-purity silicon. It is positioned as an innovative photovoltaic generation technology under the 2008 Technology Strategy Map by METI and is expected to be fully commercialized and significantly reduce the cost of producing solar cells.
Ruthenium complexes contained in conventional DSSCs are not used in the new solar cell, thus meeting the requirement for conserving this rare metal. In addition, the use of ionic liquid (IL)-based electrolyte in the cell has led to a good long-term stability of more than 2000 hours under AM 1.5 G irradiation (100 mW cm-2), compared with that less than 100 hours in DSSCs with volatile organic solvent based electrolytes.
Furthermore, this DSSC (a type of IL-based electrolyte(Note 2)) achieved a high solar-to-electricity conversion efficiency (cell efficiency) of 7.6% under AM 1.5 G irradiation, which is one of the highest performance among DSSCs with IL based electrolytes, and 5.5% efficiency with ionic gel-based electrolyte(Note 3). It is expected that the cell will be commercialized as an innovative solar generation technology.
Note 1) MK-2 refers to 2-Cyano-3-[5’’’-(9-ethyl-9H-carbazol-3-yl)-3’,3’’,3’’’,4-tetra-n-hexyl-[2,2’,5’,2’’,5’’,2’’’]-quarter thiophenyl-5-yl] acrylic acid. It is a donor-acceptor type organic dye molecule that consists of carbazol, oligo hexylthiophene and cyano acrylic acid. Its carbazole structure and cyano acrylic acid function as electron donor and acceptor, respectively. [back]
Note 2) Ionic liquid-based electrolyte refers to ionic liquid such as imidazolium iodides and iodine redox ion-based electrolyte. [back]
Note 3) Ionic gel-based electrolyte refers to the quasi-solid electrolyte generated after the addition of gellant (e.g., poly(pyridinium-1,4-diyliminocarbonyl-1,4-phenylene-methylene iodide), etc.) to the ionic liquid-based electrolyte. [back]
Research and development has been geared towards commercialization of a next-generation DSSC that reduces the environmental load. Conventional DSSCs, however, employ ruthenium complex for the photo absorption material, and consumption of this rare metal may soon be restricted in terms of shortage of resource and increased cost. Furthermore, due to the use of iodine redox electrolytes containing volatile organic solvents (containing iodine and iodide ion), achieving longer durability presented a challenge to us.
In this project, in order to increase the efficiency of conventional cells and solve the above-mentioned issues at the same time, we developed a new organic dye photo absorption material (MK-2) as an alternative to the ruthenium complex and also a new organic dye solar cell using a gelator with an organic electrolyte oligomer(Note 4) structure (results of research on the development of a new, easily synthesized electrolyte gelator and the development of a high-function hybrid gel based on the gelator under the 2005 Second Grants-in-Aid Program for Scientific Research (Masaru Yoshida, AIST)).
For development of the new organic dye, the dye was optimized with the aid of molecular design technology. Although coumarin dyes(Note 5) provided high efficiencies of up to 8% with the using volatile organic solvent based electrolyte, the efficiency of electron transfer from the dye to the titanium oxide electrodes was lower and the electron life was shorter, decreasing the solar-cell performance. Consequently, an MK dye (carbazole dye(Note 6)) was synthesized to solve this problem.
In addition, a combination of IL-based and ionic gel-based electrolytes enabled a highly efficient and suitably durable cell.
Note 4) Organic electrolyte oligomer molecules have a structure of several linked organic salt monomers. The number of organic salt monomers was relatively small, 3 to 30. [back]
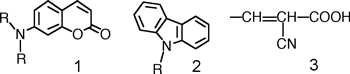
Note 5) Coumarin dyes are organic dye molecules that make the coumarin structure function as an electron donor, and make it link to cyano acrylic acid as an electron receptor (see Fig. 4). [back]
Note 6) Carbazol dyes are organic dye molecules that make the carbazol structure function as an electron donor, and make it link to cyano acrylic acid as an electron receptor (see Fig. 4). [back]
 |
|
Fig. 4 Structure of coumarin structure (1), carbazole structure (2) and cyano acrylic acid (3) (R is a substituent) |
In energy conversion efficiency, our goal is to attain 18% cell efficiency and 15% module efficiency (equivalent to the efficiency of crystalline silicon) (dye-sensitized solar cell's target value in 2030 under NEDO's solar generation roadmap PV 2030). In the immediate future, AIST intends to improve the cell's efficiency and durability with the aim of early commercialization for indoor application. We will engage in molecular design and synthesis of new organic dyes and R&D of ionic liquids and gel electrolytes, and work with our partner company to develop new electrode materials.
We will also promote joint research on technologies for production of large-area modules towards their commercialization.